Difference between revisions of "Procedure for Capillary Electrophoresis with C4D"
(→Procedure) |
(→Procedure) |
||
| (31 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | {{#ev:youtube|BtzbIXRy9lM|300|right|Video for a Procedure for C4D Analysis in PowerChrom Software}} | ||
''A procedure for capillary electrophoresis with C4D detection using the PowerChrom software is described.'' | ''A procedure for capillary electrophoresis with C4D detection using the PowerChrom software is described.'' | ||
| Line 5: | Line 6: | ||
== Equipment Required == | == Equipment Required == | ||
| − | * C4D hardware unit: | + | * C4D hardware unit, either: |
| − | ** ER225 C4D Data System | + | ** [http://www.edaq.com/ER225 ER225 C4D Data System] including [http://www.edaq.com/ES280 PowerChrom software] or |
| − | ** | + | ** [http://www.edaq.com/ER815 ER815 C4D Detector] with either a third-party data acquisition system or PowerChrom software. |
| − | * ET120 Headstage | + | * [http://www.edaq.com/ET120 ET120 C4D Headstage]. |
| − | * | + | * [Http://www.edaq.com/EC020 EC020 Standard Test Solutions for C4D]: |
| − | ** BGE = 0.5M acetic acid | + | ** BGE = 0.5M acetic acid. |
| − | ** Sample = 1mM LiCl, | + | ** Sample = 1mM LiCl, KNO<sub>3</sub> and Na<sub>2</sub>SO<sub>4</sub> in deionised water. |
| − | * Computer with the latest PowerChrom software. | + | * Computer with the latest [http://www.edaq.com/ES280 PowerChrom software]. |
* Capillary electrophoresis instrument. | * Capillary electrophoresis instrument. | ||
* Instruction manual to the CE instrument. | * Instruction manual to the CE instrument. | ||
| − | * Instruction manual to the C4D | + | * Instruction [https://www.edaq.com/edaq-product-manuals manual] to the C4D hardware and software. |
| − | * Application note showing how to connect the C4D to your CE instrument. | + | * Application note found [https://www.edaq.com/wiki/Application_Notes#C4D_Conductivity_Detector here] showing how to connect the C4D to your CE instrument. |
| + | |||
| + | == Conditions == | ||
| + | The results in this application note were obtained using the following conditions: | ||
| + | * Separation voltage | ||
| + | ** +30 kV for cations | ||
| + | ** –30 kV for anions | ||
| + | * Capillary: | ||
| + | ** Fused-silica from Polymicro Technologies | ||
| + | ** Outer diameter = 360 μm | ||
| + | ** Internal diameter = 25 μm | ||
| + | ** Length = 65 cm | ||
| + | ** Length to detector = 50 cm | ||
| + | ** Injection: hydrodynamic 3 seconds at 50 mbar | ||
| + | * C4D settings | ||
| + | ** Frequency = 1100 kHz | ||
| + | ** Amplitude = 100 % | ||
| + | ** Headstage gain ON | ||
| + | |||
| + | The 1mM LiCl, KNO3, Na2SO4 sample was diluted to 200 μM, 100 μM, 50 μM and 25 μM with the 0.5M acetic acid BGE. | ||
== Procedure == | == Procedure == | ||
| − | # Ensure you have installed the latest version of PowerChrom software from www.edaq.com/ | + | {| |
| − | # Connect the computer to the C4D hardware, following the instructions in the | + | | [[File:Easy access.jpg|300px|thumb|right|Figure 1. Easy access window]] |
| − | # Connect the C4D | + | | [[File:Manual sampling.jpg|300px|thumb|right|Figure 2. Manual Sampling window]] |
| + | | [[File:Hardware settings.jpg|300px|thumb|right|Figure 3. Hardware Settings window]] | ||
| + | |} | ||
| + | # Ensure you have installed the latest version of PowerChrom software from [http://www.edaq.com/software-downloads Software Downloads] | ||
| + | # Connect the computer to the C4D hardware, following the instructions in the [https://www.edaq.com/edaq-product-manuals manuals]. | ||
| + | # Connect the C4D hardware unit to the third-party CE instrument: connect the trigger cable and/or signal cable, depending on whether you are using eDAQ or third-party software. Follow the instructions in the relevant [https://www.edaq.com/wiki/Application_Notes#C4D_Conductivity_Detector Application Note] | ||
| + | # Connect the C4D headstage to the capillary in the instrument. Ensure that you position the C4D headstage near the end of the capillary that is grounded, away from the end of the capillary where the high voltage is applied. This is to ensure that the high voltage doesn’t arc from the background electrolyte inside the capillary, through the thin wall of the capillary, to the headstage and on to the C4D hardware, which could damage the equipment. | ||
# Turn on the C4D hardware. | # Turn on the C4D hardware. | ||
# Open the PowerChrom software. | # Open the PowerChrom software. | ||
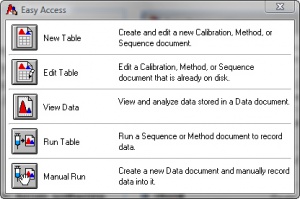
# In the Easy Access window, shown in Figure 1., click Manual Run. | # In the Easy Access window, shown in Figure 1., click Manual Run. | ||
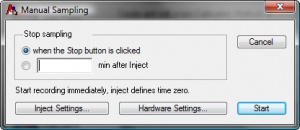
# In the Manual Sampling window, shown in Figure 2., click Hardware Settings. | # In the Manual Sampling window, shown in Figure 2., click Hardware Settings. | ||
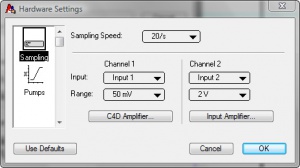
| − | # Set the following values in the Hardware Settings window, as shown in Figure 3.: | + | # Set the following values in the Hardware Settings window, as shown in Figure 3.:[[File:C4d amplifier.jpg|300px|thumb|right|Figure 4. C4D Amplifier window]][[File:Waiting for inject.jpg|300px|thumb|right|Figure 5. Waiting for Inject]] |
#* Sampling speed = 20/s | #* Sampling speed = 20/s | ||
#* Channel 1: Input = Input 1, Range = 50 mV | #* Channel 1: Input = Input 1, Range = 50 mV | ||
| − | #* Channel 2: Input = | + | #* Channel 2: Input = OFF (newer models of the ER225 are able to record a signal from an external detector, like a UV detector, in channel 2. |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
# Under Channel 1, click C4D Amplifier. | # Under Channel 1, click C4D Amplifier. | ||
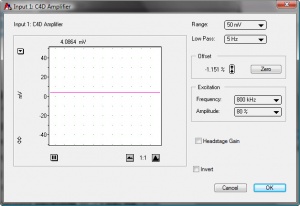
# Set the following values, as shown in Figure 4.: | # Set the following values, as shown in Figure 4.: | ||
#* Range = 50 mV | #* Range = 50 mV | ||
#* Low Pass= 5Hz | #* Low Pass= 5Hz | ||
| − | # Set the required values for Excitation Frequency and Amplitude, and set the Headstage Gain on or off. The optimum settings can be determined using C4D Profiler | + | # Set the required values for Excitation Frequency and Amplitude, and set the Headstage Gain on or off. The optimum settings can be determined using [https://www.edaq.com/ES770 C4D Profiler V2 Software]. This is described in a [https://www.edaq.com/wiki/Optimizing_the_C4D_Settings_using_the_C4D_Profiler_V2_Software separate application note]. |
# Inject your background electrolyte (BGE) into the capillary. This can done using the instrument’s controls. Alternatively, you can connect the headstage to the capillary outside the instrument, and manually inject the BGE into the capillary using a syringe connected to the capillary. | # Inject your background electrolyte (BGE) into the capillary. This can done using the instrument’s controls. Alternatively, you can connect the headstage to the capillary outside the instrument, and manually inject the BGE into the capillary using a syringe connected to the capillary. | ||
| − | # Click Zero in the Offset box. | + | # Click Zero in the Offset box. It may be best to zero the signal with the CE high voltage turned on, as this can cause a significant jump in the C4D signal. |
# Click OK to exit the C4D Amplifier window, and OK again to exit the Hardware Settings window. | # Click OK to exit the C4D Amplifier window, and OK again to exit the Hardware Settings window. | ||
| − | # Click Start | + | # Click Start. If it's a new file, enter a file name and click Save. |
| − | # Click Inject, in the Manual Sampling box (shown in Figure 5.), when the sample is injected into the capillary. | + | # Click Inject, in the Manual Sampling box (shown in Figure 5.), when the sample is injected into the capillary, unless you have installed the trigger cable to trigger automatically. |
# Click Stop, in the Manual Sampling box, when you have collected all of your peaks. | # Click Stop, in the Manual Sampling box, when you have collected all of your peaks. | ||
| − | # Repeat steps | + | # Repeat the three previous steps for each blank, calibration standard and sample you wish to analyse. |
| − | # Analyse the data, by integrating the peaks and drawing a calibration graph, as shown in the PowerChrom software | + | # Analyse the data, by integrating the peaks and drawing a calibration graph, as shown in the PowerChrom software [https://www.edaq.com/edaq-product-manuals manual] and the [https://www.edaq.com/wiki/Screencast_Training_Videos#PowerChrom screencast training videos] |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
== Results == | == Results == | ||
| − | + | [[File:Figure 8. Calibration curves for potassium and chloride.jpg|x200px|thumb|right|Figure 8. Calibration curves for potassium and chloride.]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
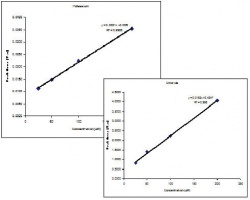
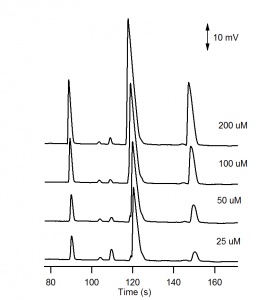
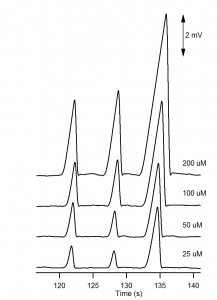
The electropherograms for the cations and anions are shown in Figure 6. and Figure 7, respectively. The calibration curves for potassium and chloride, shown in Figure 8., show good linearity. | The electropherograms for the cations and anions are shown in Figure 6. and Figure 7, respectively. The calibration curves for potassium and chloride, shown in Figure 8., show good linearity. | ||
| − | For the analysis of the cations, three negative peaks are recorded. The peaks are negative because the three cations Li+, | + | For the analysis of the cations, three negative peaks are recorded. The peaks are negative because the three cations Li<sup>+</sup>, Na<sup>+</sup> and K<sup>+</sup> are less conductive than the H<sup>+</sup> cation they are displacing in the background electrolyte. The electropherogram can be recorded with positive peaks (as in Figure 6) by selecting Invert in the C4D Amplifier window. |
{| | {| | ||
| [[File:Basel cations.jpg|x300px|thumb|right|Figure 6. Electropherograms showing cations.]] | | [[File:Basel cations.jpg|x300px|thumb|right|Figure 6. Electropherograms showing cations.]] | ||
| [[File:Basel anions.jpg|x300px|thumb|right|Figure 7. Electropherograms showing anions.]] | | [[File:Basel anions.jpg|x300px|thumb|right|Figure 7. Electropherograms showing anions.]] | ||
| − | |||
|} | |} | ||
Latest revision as of 15:47, 31 May 2018
A procedure for capillary electrophoresis with C4D detection using the PowerChrom software is described.
Introduction
This application note describes a step-by-step procedure for the analysis of the EC20 Standard Test solutions by capillary electrophoresis with capacitively-coupled contactless conductivity detection (CE-C4D). The PowerChrom software from eDAQ is used to record and analyse the data.
Equipment Required
- C4D hardware unit, either:
- ER225 C4D Data System including PowerChrom software or
- ER815 C4D Detector with either a third-party data acquisition system or PowerChrom software.
- ET120 C4D Headstage.
- EC020 Standard Test Solutions for C4D:
- BGE = 0.5M acetic acid.
- Sample = 1mM LiCl, KNO3 and Na2SO4 in deionised water.
- Computer with the latest PowerChrom software.
- Capillary electrophoresis instrument.
- Instruction manual to the CE instrument.
- Instruction manual to the C4D hardware and software.
- Application note found here showing how to connect the C4D to your CE instrument.
Conditions
The results in this application note were obtained using the following conditions:
- Separation voltage
- +30 kV for cations
- –30 kV for anions
- Capillary:
- Fused-silica from Polymicro Technologies
- Outer diameter = 360 μm
- Internal diameter = 25 μm
- Length = 65 cm
- Length to detector = 50 cm
- Injection: hydrodynamic 3 seconds at 50 mbar
- C4D settings
- Frequency = 1100 kHz
- Amplitude = 100 %
- Headstage gain ON
The 1mM LiCl, KNO3, Na2SO4 sample was diluted to 200 μM, 100 μM, 50 μM and 25 μM with the 0.5M acetic acid BGE.
Procedure
- Ensure you have installed the latest version of PowerChrom software from Software Downloads
- Connect the computer to the C4D hardware, following the instructions in the manuals.
- Connect the C4D hardware unit to the third-party CE instrument: connect the trigger cable and/or signal cable, depending on whether you are using eDAQ or third-party software. Follow the instructions in the relevant Application Note
- Connect the C4D headstage to the capillary in the instrument. Ensure that you position the C4D headstage near the end of the capillary that is grounded, away from the end of the capillary where the high voltage is applied. This is to ensure that the high voltage doesn’t arc from the background electrolyte inside the capillary, through the thin wall of the capillary, to the headstage and on to the C4D hardware, which could damage the equipment.
- Turn on the C4D hardware.
- Open the PowerChrom software.
- In the Easy Access window, shown in Figure 1., click Manual Run.
- In the Manual Sampling window, shown in Figure 2., click Hardware Settings.
- Set the following values in the Hardware Settings window, as shown in Figure 3.:
- Sampling speed = 20/s
- Channel 1: Input = Input 1, Range = 50 mV
- Channel 2: Input = OFF (newer models of the ER225 are able to record a signal from an external detector, like a UV detector, in channel 2.
- Under Channel 1, click C4D Amplifier.
- Set the following values, as shown in Figure 4.:
- Range = 50 mV
- Low Pass= 5Hz
- Set the required values for Excitation Frequency and Amplitude, and set the Headstage Gain on or off. The optimum settings can be determined using C4D Profiler V2 Software. This is described in a separate application note.
- Inject your background electrolyte (BGE) into the capillary. This can done using the instrument’s controls. Alternatively, you can connect the headstage to the capillary outside the instrument, and manually inject the BGE into the capillary using a syringe connected to the capillary.
- Click Zero in the Offset box. It may be best to zero the signal with the CE high voltage turned on, as this can cause a significant jump in the C4D signal.
- Click OK to exit the C4D Amplifier window, and OK again to exit the Hardware Settings window.
- Click Start. If it's a new file, enter a file name and click Save.
- Click Inject, in the Manual Sampling box (shown in Figure 5.), when the sample is injected into the capillary, unless you have installed the trigger cable to trigger automatically.
- Click Stop, in the Manual Sampling box, when you have collected all of your peaks.
- Repeat the three previous steps for each blank, calibration standard and sample you wish to analyse.
- Analyse the data, by integrating the peaks and drawing a calibration graph, as shown in the PowerChrom software manual and the screencast training videos
Results
The electropherograms for the cations and anions are shown in Figure 6. and Figure 7, respectively. The calibration curves for potassium and chloride, shown in Figure 8., show good linearity.
For the analysis of the cations, three negative peaks are recorded. The peaks are negative because the three cations Li+, Na+ and K+ are less conductive than the H+ cation they are displacing in the background electrolyte. The electropherogram can be recorded with positive peaks (as in Figure 6) by selecting Invert in the C4D Amplifier window.
Notes
It is generally recommend to use a capillary with a small inner diameter such as 25 µm. Capillaries with larger inner diameters can lead to greater Joule heating and unstable baselines.
Dissolving in water leads to a difference in conductivity during loading, between the sample and the BGE in capillary. This produces an electrokinetic bias, where faster migrating compounds are introduced into the capillary in a greater quantity than slower migrating compounds.
If the volume of BGE in the EC20 Standard Test Solutions is not enough to rinse the capillary and perform the experiment, more 0.5M acetic acid can easily be made up by the user.