Ionophores in Society
Medicines, Toxins, Pollutants, Growth Promoters, Sensors
Ionophores are lipophilic molecules produced, and often excreted, by microorganisms and animals that are characterised by their ability to transport ions (especially Na+, K+, Ca2+, and Mg2+) across cell membranes. They are not only chemically interesting in their own right but many have important medical and economic roles in our society.
A selection of ionophores is presented below for study in mainstream chemistry courses as well as courses for pre-med, vet, pharma, biochemistry, and soil and agricultural science students. The demonstration of ion movement across membranes is also relevant to courses on neurophysiology, and biophysics.
The use of ionophore 'growth promoters' in the animal feedstock industry is also of concern to environmental chemists because of their presence in manure and sewerage sludges used as fertiliser for human and animal crops.
The ability of these materials to transport ions across phospholipid bilayer cell membranes, which is usually the key to understanding their mode of action, can be easily studied using tethered membrane technology. A suitable method is outlined in Application Note: SDx Protocol 1.
Most of these ionophores can be obtained from eDAQ.
Contents
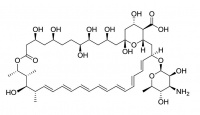
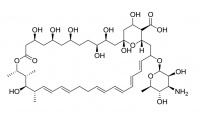
Amphotericin B
Amphotericin B is an antibiotic (AmBisome®, ) used to treat serious fungal infections. It forms ion channels in the fungal cell membrane with the assistance of ergosterol which is a naturally occurring lipid in fungal membranes. As a medicine it is administered intravenously as a liposomal preparation.
While its affinity for ergosterol makes it target fungal cells, Amphotericin can also interact, though to a lesser extent, with the cholesterol in mammalian cells. This limits its therapeutic dosage because of toxicity.
Amphotericin B is similar both in structure and in action to Nystatin.
Dissolve 25 mg in 27.0 mL of solvent to make a 1.00 mM stock solution.
References
- Novel, unifying mechanism for amphotericin B and other polyene drugs: electron affinity, radicals, electron transfer, autoxidation, toxicity, and antifungal action. Peter Kovacic and Andrew Cooksy, Medicinal Chemical Communications, 3, 274-280, 2012. DOI: 10.1039/C2MD00267A
- Drug Information Online.
- Available from eDAQ.
Beauvericin
Beauvericin is closely related to the macrocyclic enniatins, having three benzyl groups in place of the aliphatic chains of the enniatins. It is a potentially toxic agent found in grain crops affected by Fusarium fungi. The selectivity for monovalent cations is reported as K+ > Na+ ~ Rb+ > Cs+ ~ Li+ > NH4+, and for divalent cations as Ca2+ > Sr2+ > Ba2+ > Mg2+. The antibiotic properties of Beauvericin are being investigated.
Dissolve 25 mg in 31.9 mL of solvent to make a 1.00 mM stock solution.
References
- Occurrence of Beauvericin and Enniatins in Wheat Affected by Fusarium avenaceum Head Blight. A Logrieco, A Rizzo, R Ferracane, and A Ritieni, Applied Environmental Microbiology, 68, 82-85, 2002. DOI: 10.1128/AEM.68.1.82-85.2002
- Selective Binding of Alkali and Alakaline Earth Ions to Beauvericin Investigated by Electrospray Mass Spectrometry. Tara-Lynn Baratz, Courtney N. Richardson, J. Brett Kimbrell, Joshua Hite, (Dr. Farooq A. Khan). Poster presentation, 2010.
- Beauvericin and enniatins H, I and MK1688 are new potent inhibitors of human immunodeficiency virus type-1 integrase. CG Shin, DG An, HH Song, C Lee, The Journal of antibiotics, 62, 687-690, 2009. DOI: 10.1038/ja.2009.102
- Available from eDAQ.
Calcimycin
Calcimycin is also widely known as Ionophore A23187. The most notable feature of calcimycin is the ability to transport divalent cation across cell membranes. Its in vitro affinity for cations is Ni2+ > Co2+ > Zn2+ > Mn2+ > Mg2+ ~ Ca2+ > Sr2+ > Ba2+, however transport rates across lipsomal membranes are reported as Cd2+ > Zn2+ > Ca2+ > Mg2+ > Sr2+ > Ba2+. Calcimycin has antibiotic properties, and is also being used in IVF treatments.
Dissolve 5 mg in 9.55 mL of solvent to make a 1.00 mM stock solution.
References
- Structures and Properties of Naturally Occurring Polyether Antibiotics. Jacek Rutkowski and Bogumil Brzezinski, BioMed Research International, Article ID 162513, 2013. DOI: 10.1155/2013/162513
- Equilibria between ionophore A23187 and divalent cations: stability of 1:1 complexes in solutions of 80% methanol/water. CJ Chapman, AK Puri, RW Taylor, DR Pfeiffer, Biochemistry, 26, 5009-5018, 1987. DOI: 10.1021/bi00390a019
- The specificity of ionophore A23187 in cation transport across lipid membranes. Studies with lecithin vesicles. WG Pohl, R Kreikenbohm, K. Seuwen, Z Naturforsch C, 35, 562-568, 1980.
- Ca2+ transport properties of ionophores A23187, ionomycin, and 4-BrA23187 in a well defined model system. W.L. Erdahl, C.J. Chapman, R.W. Taylor and D.R. Pfeiffer, Biophysical Journal, 66, 1678-1693, 1994. 10.1016/S0006-3495(94)80959-2
- Application of a ready-to-use calcium ionophore increases rates of fertilization and pregnancy in severe male factor infertility. Thomas Ebner, Maria Köster, Omar Shebl, Marianne Moser, Hans Van der Ven, Gernot Tews, Markus Montag, Fertility and Sterility, 98, 1432–1437, 2012. DOI: 10.1016/j.fertnstert.2012.07.1134. Free download.
- Available from eDAQ.
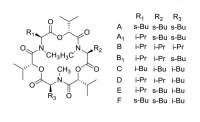
Enniatin complex
Enniatins are produced by Fusarium fungi that commonly infect wheat and other grains. High levels are potentially toxic.
A mixture of related macrocylic molecules, with different peripheral alkyl substituents, is commonly encountered. At last count there were 28 enniatins known.
There are reports of Enniantins having promise as antibiotics and anti-cancer drugs.
Dissolve 50 mg in 78.1 mL, or 10 mg in 15.6 mL, of solvent to make a 1.00 mM stock solution.
References
- Occurrence of Beauvericin and Enniatins in Wheat Affected by Fusarium avenaceum Head Blight. A Logrieco, A Rizzo, R Ferracane, and A Ritieni, Applied Environmental Microbiology, 68, 82-85, 2002. DOI: 10.1128/AEM.68.1.82-85.2002
- Beauvericin and enniatins H, I and MK1688 are new potent inhibitors of human immunodeficiency virus type-1 integrase. CG Shin, DG An, HH Song, C Lee, The Journal of antibiotics, 62, 687-690, 2009. DOI: 10.1038/ja.2009.102
- Toxicology Portal at Toxicology.no.
- Available from eDAQ.
Gramicidin
Gramicidin is often regarded as the archetypal ion channel for monovalent cations, despite its unusual dimer arrangement that can form across the cell membrane. It was one of the first commercially available antibiotics and is used today in several topical medications (Neosporin, Neocidin, Neoptic, Ocu-Spore-G).
Gramicidin is usually sold as a mixture of related linear polypeptides (principally A, B and C) but the main component is Gramicidin A.
Dissolve 100 mg in 53.1 mL of solvent to make a 1.00 mM stock solution.
Gramicidin S is an unusual macrocyclic variant.
References
- The gramicidin ion channel: A model membrane protein. Devaki A. Kelkar, Amitabha Chattopadhyay, Biochimica et Biophysica Acta 1768, 2011–2025, 2007. DOI: 10.1016/j.bbamem.2007.05.011
- Structure of gramicidin A. B A Wallace, Biophysics Journal, 49, 295–306, 1986. Free access at PubMed Central: PMC1329637
- Drug Information Online.
- Available from Sigma-Aldrich or Santa Cruz Biotechnology
Ionomycin
Ionomycin is a noted calcium ionophore with a selectivity of Pb2+ > Cd2+ > Zn2+ > Mn2+ > Ca2+ > Mg2+ >> Sr2+ ~ Ba2+. There is almost no binding to Sr2+ or Ba2+ or to monovalent alkali metal cations.
Dissolve 5 mg in 6.69 mL of solvent to make a 1.00 mM stock solution.
References
- Characterization of ionomycin as a calcium ionophore. C Liu and T E Hermann, Journal of Biological Chemistry, 253, 5892-5894, 1978.
- Ca2+ transport properties of ionophores A23187, ionomycin, and 4-BrA23187 in a well defined model system. W.L. Erdahl, C.J. Chapman, R.W. Taylor and D.R. Pfeiffer, Biophysical Journal, 66, 1678-1693, 1994. DOI:10.1016/S0006-3495(94)80959-2
- Ionomycin, a Carboxylic Acid Ionophore, Transports Pb2+ with High Selectivity. Warren L. Erdahl, Clifford J. Chapman, Richard W. Taylor and Douglas R. Pfeiffer, Journal of Biological Chemistry, 275, 7071-7079, 2000. DOI: 10.1074/jbc.275.10.7071
- Ion-selective electrodes based on natural carboxylic polyether antibiotics. Koji Suzuki, Koji Tohda, Hiroshi Aruga, Misako Matsuzoe, Hidenari Inoue, Tsuneo Shirai, Analytical Chemistry, 60, 1714–1721, 1988. DOI: 10.1021/ac00168a016
- Available from eDAQ, Hölzel Diagnostica, or LC Labs.
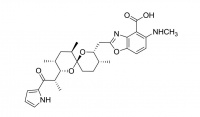
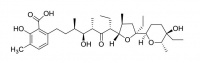
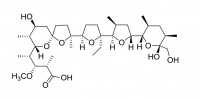
Lasalocid
Lasalocid is used as a food supplement antibiotic (Avatec®, Bovatec®) for control of coccidiosis, a protozoan parasite of poultry and livestock.
Dissolve 25 mg in 42.3 mL of solvent to make a 1.00 mM stock solution.
References
- Effect of Monensin and Lasalocid-Sodium on the Growth of Methanogenic and Rumen Saccharolytic Bacteria. Min Chen and MJ Wolin, Applied and Environmental Microbiology, 72-77, 1979.
- Ionophores for Dairy Cattle: Current Status and Future Outlook. R.K. McGuffey, L.F. Richardson, J.I.D. Wilkinson, Journal of Dairy Science 84, E194–E203, 2001. DOI: 10.3168/jds.S0022-0302(01)70218-4
- Trace Level Determination of Polyether Ionophores in Feed. BioMed Research International, 2013, Article ID 151363. Mervi Rokka, Marika Jestoi, and Kimmo Peltonen, DOI: 10.1155/2013/151363
- Ion-selective electrodes based on natural carboxylic polyether antibiotics. Koji Suzuki, Koji Tohda, Hiroshi Aruga, Misako Matsuzoe, Hidenari Inoue, Tsuneo Shirai, Analytical Chemistry, 60, 1714–1721, 1988. DOI: 10.1021/ac00168a016
- Rotation programmes for coccidiosis control. Dr H. D. Chapman, International Poultry Production, 15, 7-9, 2007. Free download.
- Drug Information Online.
- Available from eDAQ.
Maduramicin
Maduramicin is used as a food supplement antibiotic (Cygro®) for control of coccidiosis, a protozoan parasite of poultry and livestock.
Dissolve 25 mg in 27.3 mL of solvent to make a 1.00 mM stock solution.
References
- Trace Level Determination of Polyether Ionophores in Feed. BioMed Research International, 2013, Article ID 151363. Mervi Rokka, Marika Jestoi, and Kimmo Peltonen, DOI: 10.1155/2013/151363
- Rotation programmes for coccidiosis control. Dr H. D. Chapman, International Poultry Production, 15, 7-9, 2007. Free download.
- Drug Information Online.
- Available from eDAQ.
Magainin and PGLa
The magainins are linear polypeptides of 23 amino acids first discovered in the skin of the African clawed frog, Xenopus laevis, and are believed to be responsible for the resistance of the frog to many of the pathogens that would otherwise cause disease in their moist habitat. Magainins and similar compounds are now known to be present in the skin of many amphibians and are have been shown to be active against viruses, both gram-positive and gram-negative bacteria, protozoa, yeasts and fungi, and may also be hemolytic and cytotoxic to cancer cells. Their mode of action is believed to be by 4 − 7 maginin molecules forming a pore in the phospholipid bilayer membrane of their target. Magainin 2 also has a synergistic effect with the linear polypeptide PGLa (also from Xenopus) suggesting the formation of a hybrid pore.
Magainins 1 and 2 differ in two amino acid locations where a positively charged lysine residue (Lys) is replaced by a neutral amino acid.
| Magainin 1: | H-Gly-Ile-Gly-Lys-Phe-Leu-His-Ser-Ala-Gly-Lys-Phe-Gly-Lys-Ala-Phe-Val-Gly-Glu-Ile-Met-Lys-Ser-OH | MW 2410 | 1 mg in 415 µL = 1 mM |
|---|---|---|---|
| Magainin 2: | H-Gly-Ile-Gly-Lys-Phe-Leu-His-Ser-Ala-Lys-Lys-Phe-Gly-Lys-Ala-Phe-Val-Gly-Glu-Ile-Met-Asn-Ser-OH | MW 2467 | 1 mg in 405 µL = 1 mM |
| PGLa: | H-Gly-Met-Ala-Ser-Lys-Ala-Gly-Ala-Ile-Ala-Gly-Lys-Ile-Ala-Lys-Val-Ala-Leu-Lys-Ala-Leu-NH2 | MW 1969 | 1 mg in 508 µL = 1 mM |
Nonetheless at physiological pH each magainin still contains four positively charged lysine residues which are believed to help target the negatively charged cell membranes of bacteria while ignoring relatively uncharged vertebrate cell membranes. PGLa is a slightly smaller polypeptide but also has four positively charged lysine residues.
At least one derivative of magainin, Pexiganan, has been in clinical trials for use in antibiotic medications such as Locilex™.
References
- Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Michael Zasloff, Proceedings of the National Academy of Sciences, 84, 5449-5453, 1987.
- Membrane Pores Induced by Magainin. Steve J. Ludtke, Ke He, William T. Heller, Thad A. Harroun, Lin Yang, and Huey W. Huang, Biochemistry, 35, 13723-13728, 1996. DOI: 10.1021/bi9620621 Free download.
- Interaction of a Magainin−PGLa Hybrid Peptide with Membranes: Insight into the Mechanism of Synergism. Minoru Nishida , Yuichi Imura, Megumi Yamamoto, Satoe Kobayashi, Yoshiaki Yano, and Katsumi Matsuzaki, Biochemistry, 46, 14284–14290 2007. DOI: 10.1021/bi701850m
- In Vitro Antibacterial Properties of Pexiganan, an Analog of Magainin. Yigong Ge, Dorothy L. MacDonald, Kenneth J. Holroyd, Clyde Thornsberry, Hannah Wexler, and Michael Zasloff, Antimicrobial Agents and Chemotherapy, 43, 782-788, 1999. Free download.
- Magainin 1 and Magainin 2 available from AnaSpec, as well as Abbiotec and GenScript who also supply PGLa.
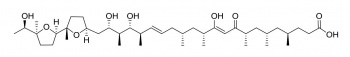
Monensin A
Monensin is used in the livestock industry as a food supplement antibiotic (Rumensin®, Coban®), where it can alter the gut flora (bacteria populations) of the animal so that animal growth rates are improved. However some species have a low tolerance and it is particularly toxic to horses.
It has been reported to have anti-tumor properties, and has also been investigated as a chelation agent for the treatment of lead toxicity.
Dissolve 25 mg in 37.3 mL of solvent to make a 1.00 mM stock solution.
References
- Structure and Antimicrobial Properties of Monensin A and Its Derivatives: Summary of the Achievements Daniel Łowicki and Adam Huczyński, BioMed Research International, Article ID 742149, 2013. DOI: 10.1155/2013/742149
- Effect of Monensin and Lasalocid-Sodium on the Growth of Methanogenic and Rumen Saccharolytic Bacteria. Min Chen and MJ Wolin, Applied and Environmental Microbiology, 72-77, 1979. Free Access at PubMed Central: PMC243437
- Ionophores for Dairy Cattle: Current Status and Future Outlook. R.K. McGuffey, L.F. Richardson, J.I.D. Wilkinson, Journal of Dairy Science 84, E194–E203, 2001. DOI: 10.3168/jds.S0022-0302(01)70218-4
- Polyether ionophores — promising bioactive molecules for cancer therapy. Adam Huczyński, Bioorganic & Medicinal Chemistry Letters, 22, 7002–7010, 2012. DOI: 10.1016/j.bmcl.2012.09.046
- Trace Level Determination of Polyether Ionophores in Feed. BioMed Research International, 2013, Article ID 151363. Mervi Rokka, Marika Jestoi, and Kimmo Peltonen, DOI: 10.1155/2013/151363
- Monensin Improves the Effectiveness of meso-Dimercaptosuccinate when Used to Treat Lead Intoxication in Rats. Shawn A. Hamidinia, Warren L. Erdahl, Clifford J. Chapman, Gregory E. Steinbaugh, Richard W. Taylor, and Douglas R. Pfeiffer, Environmental Health Perspectives, 114, 484–493, 2006. DOI: 10.1289/ehp.8279
- Rotation programmes for coccidiosis control. Dr H. D. Chapman, International Poultry Production, 15, 7-9, 2007. Free download.
- Hazard Analysis Critical Control Point (HACCP) Manual, Bio Agri Mix LP.
- Drug Information Online.
- Available from eDAQ.
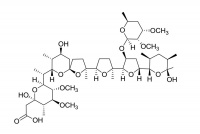
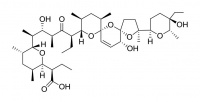
Narasin
Narasin is a methylated derivative of salinomycin, and has similar properties. It is used as a food additive (Monteban®) in the poultry industry where it acts as a coccidiostat.
Dissolve 25 mg in 32.7 mL of solvent to make a 1.00 mM stock solution.
Reference
- Trace Level Determination of Polyether Ionophores in Feed. BioMed Research International, 2013, Article ID 151363. Mervi Rokka, Marika Jestoi, and Kimmo Peltonen, DOI: 10.1155/2013/151363
- Rotation programmes for coccidiosis control. Dr H. D. Chapman, International Poultry Production, 15, 7-9, 2007. Free download.
- Drug Information Online.
- Available from eDAQ.
Nystatin A
Nystatin is an antifungal antibiotic often prescribed for the treatment of Candida infections. It forms ion channels in the fungal cell membrane with the assistance of ergosterol which is a naturally occurring lipid in fungal membranes. As a medicine Nystatin is taken orally or topically.
It is also used as an ingredient in several topical veterinary medications (Panolog®, Animax, Derma-Vet™, Panavet and Derm-Otic) for cats and dogs, and as a food supplement antibiotic (Pharmastatin®-20) for Candida infections in turkeys.
While its affinity for ergosterol makes it target fungal cells, it can also interact, though to a lesser extent, with the cholesterol in mammalian cells. This can limit its therapeutic dosage because of toxicity.
It is similar both in structure and in action to Amphotericin B.
Leafcutter ants use nystatin-like antibiotics to selectively raise the fungus they produce in their leaf-mulch gardens.
Dissolve 25 mg in 27.0 mL of solvent to make a 1.00 mM stock solution.
References
- Drug Information Online.
- A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. Jörg Barke, Ryan F. Seipke, Sabine Grüschow, Darren Heavens, Nizar Drou, Mervyn J. Bibb,Rebecca J.M. Goss, Douglas W. Yu, Matthew I. Hutchings, BMC Biology, 8, 109, 2010. DOI: 10.1186/1741-7007-8-109
- Available from eDAQ.
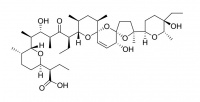
Salinomycin
Salinomycin is used in the poultry industry as a food supplement (Bio-Cox®, Sacox® ), where it acts as a coccidiostat.
It is also being investigated as an anti-cancer agent.
Dissolve 25 mg in 33.3 mL of solvent to make a 1.00 mM stock solution.
References
- Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Michael P. Schlüsener, Kai Bester, Environmental Pollution, 143, 565–571, 2006. DOI: 10.1016/j.envpol.2005.10.049
- Effects of the veterinary pharmaceutical salinomycin and its formulation on the plant Brassica rapa. V. Furtula, G. L. Stephenson, K. M. Olaveson, P. A. Chambers, Archives of Environmental Contamination and Toxicology, 63, 513-522, 2012. DOI: 10.1007/s00244-012-9807-y
- Polyether ionophores—promising bioactive molecules for cancer therapy. Adam Huczyński, Bioorganic & Medicinal Chemistry Letters, 22, 7002–7010, 2012. DOI: 10.1016/j.bmcl.2012.09.046
- Trace Level Determination of Polyether Ionophores in Feed. BioMed Research International, 2013, Article ID 151363. Mervi Rokka, Marika Jestoi, and Kimmo Peltonen, DOI: 10.1155/2013/151363
- A case of human poisoning by salinomycin, an agricultural antibiotic. Phillipa Story and Alan Doube, The New Zealand Medical Journal, 117, 1190, 2004.
- Rotation programmes for coccidiosis control. Dr H. D. Chapman, International Poultry Production, 15, 7-9, 2007.
- Hazard Analysis Critical Control Point (HACCP) Manual, Bio Agri Mix LP.
- SACOX Product Information Sheet, Invert Inc.
- Drug Information Online.
- Available from eDAQ.
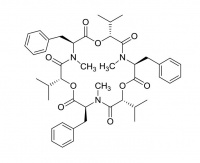
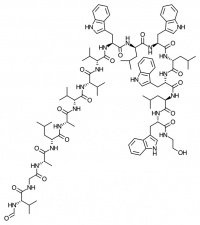
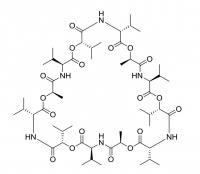
Valinomycin
Valinomycin is a macrocylic polypeptide and is historically one of the best known ionophores, especially for its 104 fold selectivity for potassium over sodium ions. It is being investigated for use as an antibiotic, as well as being used in the manufacture of potassium ion selective electrodes.
Dissolve 100 mg in 90.0 mL of solvent to make a 1.00 mM stock solution.
References
- K+/Na+ Selectivity in K-Channels and Valinomycin: Over-coordination Versus Cavity-size constraints. Sameer Varma, Dubravko Sabo, Susan B. Rempe, Journal of Molecular Biology, 376 13–22, 2008. DOI: 10.1016/j.jmb.2007.11.059. Free Access at PubMed Central: PMC2390915
- Design principles for K+ selectivity in membrane transport. Sameer Varma, David M. Rogers, Lawrence R. Pratt, and Susan B. Rempe, Journal of General Physiology, 137, 479-488, 2011. DOI: 10.1085/jgp.201010579
- Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes, Philippe Bühlmann and Li D. Chen, 2539-2579 in "Supramolecular Chemistry: From Molecules to Nanomaterials", Philip A. Gale and Jonathan W. Steed (Eds), John Wiley & Sons Ltd. 2012. ISBN: 978-0-470-74640-0. Free download.
- Available from eDAQ.