Method for Counting the Total Number of Colonies in Ice Cream
1 Scope
This standard outlines the methodology for determining the total count of colonies of both aerobic and facultative anaerobic bacteria in ice cream.
2 Normative references
This standard references certain provisions contained in the reference standard. For dated citation standards, subsequent changes or revisions will not apply. However, parties who agree to follow this standard are encouraged to consider using the latest version of the reference standard described below.GB 4789.2-2016 Food Microbiological Analysis - Total Colonies Count
3 Principle of testing
During microbial growth, large molecules with low electrical conductivity present in the medium undergo metabolic processes that transform them into small molecules and ions with higher electrical conductivity, such as amino acids. As a result, the conductivity of the medium changes. The detectable time for this change is inversely proportional to the logarithm of the initial microbial concentration. This allows for the determination of the concentration and quantity of culturable bacteria in the sample to be tested through the use of a working curve.
4 Equipment and materials
In addition to the routine sterilization and culture equipment in the microbiology laboratory, other equipment and materials are as follows:
4.1 16/32 channels CellStatz electronic microbial growth analyzer
4.2 Sterile scalpel
4.3 Micropipette and suction head: the measuring ranges are 100 μL, 1 mL and 5 mL, respectively
4.4 Scale: accuracy is 0.0001 g
4.5 Constant Temperature Incubator: 36 ℃±1 ℃, 30 ℃±1 ℃
4.6 Table Concentrator: ±1 ℃
4.7 Fridge: 2 ℃~5 ℃,-18℃~-20℃
4.8 Sterile petri dish, OD 90 mm
5 Medium and reagent
5.1 LB liquid medium: Details see A1
5.2 Plate counting AGAR medium: Details see A2
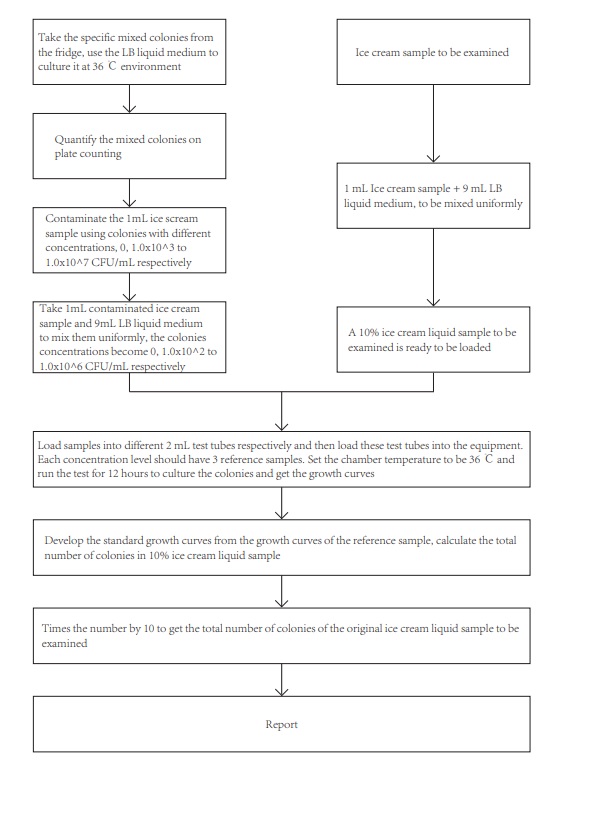
6 Test procedure
See block diagram shown below:
7 Detailed test procedure
7.1 Develop the standard growth curves for the total number of colonies
7.1.1 To begin the experiment, a 100 μL aliquot of a specific mixed bacterial flora solution stored at -20℃ should be taken and inoculated into 4 mL of LB liquid medium. The culture should then be incubated overnight at 36℃ with shaking at 180 r/min.
7.1.2 The concentration of the activated bacterial solution will be determined by employing the plate counting method described in GB 4789.2-2016.
7.1.3 Following quantification of the bacterial solution, different concentration gradients will be established for the contamination of 1 mL of ice cream solution. Specifically, the bacterial concentration of the contaminated ice cream solution will be set at 1.0x10^7, 1.0×10^6, 1.0×10^5, 1.0×10^4, 1.0×10^3, 1.0×10^2, 1.0×10^1, and 0 CFU/mL. Subsequently, 1 mL of each contaminated ice cream solution will be mixed uniformly with 9 mL of LB liquid medium to generate standard growth curves with known bacterial concentrations of 1.0×10^6, 1.0×10^5, 1.0×10^4, 1.0×10^3, 1.0×10^2, 1.0×10^1, and 0 CFU/mL.
7.2 Procedure for samples to be examined
7.2.1 To begin the analysis, an appropriate amount of the ice cream sample will be obtained using a random sampling method. The representative samples will be collected using aseptic techniques and placed in sterilized containers to allow for natural melting. In cases where the ice cream is packaged, the opening of the packaging will be swabbed with 75% ethanol before sampling to ensure sterility.
7.2.2 A 1 mL aliquot of the ice cream solution will be transferred using a pipette and added to 9 mL of LB liquid medium. The solution will then be thoroughly mixed to prepare a bacterial solution containing 10% LB medium from the ice cream sample for analysis using the designated equipment.
7.3 Negative control sample A 1 mL aliquot of sterile ice cream solution will be transferred and added to 9 mL of LB liquid medium. The mixture will be thoroughly mixed to prepare a negative control sample for analysis.
7.4 Culture the colonies
The prepared standard curve samples and the ice cream samples to be tested will be loaded into separate 2 mL test tubes. Each concentration level will have three reference samples. These test tubes will be placed into the designated equipment and incubated at 36°C for 12 hours. Conductivity measurements will be taken at 60-second intervals to obtain the necessary data.
7.5 Data processing
The data obtained from the microbial growth sensor, including electrical conductivity and culture time (T, in minutes), will be used to plot the microbial growth curve. By analyzing the relationship between the concentration of the standard bacterial solution and its corresponding detection time, a quantitative working function C bacteria (CFU/mL) = mT (min) + n will be obtained. The detection time (T) of the ice cream sample will be substituted into this function to obtain the total number of bacterial colonies present in the sample. The resulting number will be multiplied by 10 to determine the total number of bacterial colonies in the ice cream sample.
7.6 Results
7.6.1 If the total number of bacterial colonies in the samples obtained during session 7.5 is less than 100, the data shall be reported with two significant figures.
7.6.2 When reporting the total number of colonies of the samples obtained from session 7.5, the appropriate significant figures should be used based on the magnitude of the number. If the total number of colonies is less than 100, a two-digit significant number should be used. However, if the total number of colonies is greater than 100, the third digit should be rounded off and replaced by a zero. Alternatively, the number can be expressed in exponential form as a power of 10.
7.7 Test report
The test report should indicate:
- All the information necessary for the complete identification of the sample;
- Sampling method adopted, if any;
- All operational details not specified in this standard and some factors that may have affected the test results;
- The obtained result, indicating the method of representation adopted;
- Final results cited if repeated checks have been made;
- Quantitative work functions should be established separately for each type and specification of ice cream products;
- The working function needs to be validated by plate counting every six months.
8 Standard specification
This standard was completed on 6th September 2021.
Appendix A
A1 LB liquid medium
A1.1 Components
Yeast Extract 5g
Tryptone 10 g
Sodium chloride 10 g
Distilled Water 1000 mL
A1.2 Preparation
To prepare the solution, the specified ingredients should be added to distilled water, and then the mixture should be boiled and stirred until fully dissolved. The pH of the solution should be adjusted to 7.0±0.2 using an appropriate buffer solution. Next, the solution should be divided into either test tubes or conical bottles, and sterilized via autoclaving at 121 ℃ for a duration of 15 minutes.
A2 Plate count agar
A2.1 Components
Tryptone 5.0g
Yeast Extract 2.5g
Glucose 1.0g
June Fat 15.0g
Distilled Water 1000 mL
A2.2 Preparation
To prepare the solution, the specified ingredients should be added to distilled water, and then the mixture should be boiled and stirred until fully dissolved. The pH of the solution should be adjusted to 7.0±0.2 using an appropriate buffer solution. Next, the solution should be divided into either test tubes or conical bottles, and sterilized via autoclaving at 121 ℃ for a duration of 15 minutes.