Difference between revisions of "SDxプロトコール"
(→膜の形成) |
(→膜の作成) |
||
| Line 6: | Line 6: | ||
=== '''膜の作成''' === | === '''膜の作成''' === | ||
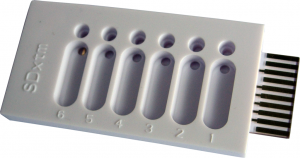
| − | [[File:tethaPlate6.png|300px|thumb|right|'''図 1.''' tethaPlate. | + | [[File:tethaPlate6.png|300px|thumb|right|'''図 1.''' tethaPlate. 各々小さな循環流入ウェルを持つ6個の小室と大きな長円形の排水ウェルが見えます。]] |
| − | + | [http://wiki.edaq.com/index.php/How_to_assemble_a_tethaPlate_cartridge 説明書] に従って[http://www.edaq.com/main.php?url=SDx-T10 tethaPlate] をアッセンブルします。 | |
| − | + | [http://wiki.edaq.com/index.php/Making_a_tethered_membrane メンブレンの作成:] | |
| − | # | + | # [http://www.edaq.com/SDx-S1 SDx-S1 リン脂質混合液,] を 10 µL 各インプットウェルに注入し、2分間インキュベートして脂質二重膜を形成させる。 |
# Add 200 µL PBS per well | # Add 200 µL PBS per well | ||
# Successively rinse three times by the addition of 200 µL PBS. | # Successively rinse three times by the addition of 200 µL PBS. | ||
Revision as of 12:52, 3 June 2014
このプロトコールは イオノフォア や低分子 and other low molecular weight (2000 Da 以下の) イオンチャネルなどを tethaPod™ システムを使って、それらのテザード膜の電導度を測定する為の手順を示したものです。測定の教材に適したイオノフォアは Ionophores in Society のアプリケーションノートに記載されています。
測定前の準備としてに テザードメンブレン のコンセプトと tethaPod ソフトウェア の使い方を習得しておいてください。
このプロトコールで使用するバッファー溶液は PBS (リン酸緩衝食塩水) 溶液です。ただ、別のプロトコールでは異なる緩衝溶液、たとえば 0.1 M KCl、リンガー液 通常の生理食塩水、その他 Good 緩衝液、 TBS など。 下記で説明する多くのイオノフォアは脂肪族カルボン酸基を持ち、pKa は約 4.5 ~ 5 を示します。これらは pH 6.5 以上では完全にイオン化し、水に優れた可溶性を示すのでpH 緩衝液として活用されています。二価の陽イオン (Mg2+、Ca2+ など) に対して特異性を示すイオノフォアを扱う際は、これらのイオンを一定濃度含む緩衝溶液が必要です。
Contents
膜の作成
説明書 に従ってtethaPlate をアッセンブルします。 メンブレンの作成:
- SDx-S1 リン脂質混合液, を 10 µL 各インプットウェルに注入し、2分間インキュベートして脂質二重膜を形成させる。
- Add 200 µL PBS per well
- Successively rinse three times by the addition of 200 µL PBS.
- Remove the excess PBS from the waste well.
In cases where ion channel formation requires the presence of ergosterol or cholesterol (eg when studying Amphotericin B or Nystatin) it is necessary to add these substances the SDx-S1 Phospholipid Mix prior to membrane formation.
溶液の調整
Ionophores are usually supplied in milligram quantities in vials or small bottles. A stock solution is usually prepared by rinsing the contents from the manufacturer's container into a larger vessel and making up a known volume of solution. Most manufacturers supply an amount of ionophore in a slightly greater quantity (by 1 − 10%) than indicated on the container's label. If exact concentrations are required, weigh the manufacturer's container before and after the contents are rinsed out, and use the determined mass in your calculations.
Make a 1 mM stock solution of the ionophore in methanol. Long term storage of this stock solution at −20°C (i.e. in a freezer) is recommended. Overnight storage, or even for several days, can be done at 2 − 4°C (ie in a refrigerator). Allow the stock solution to return to room temperature before using.
Now prepare a 10 µM (10000 nM) solution in PBS buffer by doing a 1:100 dilution of an aliquot of the stock solution. Most 'lipophillic' ionophores are either sufficiently water soluble so as to be able to achieve a 10 µM concentration, or will form a supersaturated solution long enough for you to prepare the more dilute solutions. Using the method of serial dilution to prepare solutions of 1, 10, 100, and 1000 nM in PBS buffer.
Note that the 10 µM solution will still contain only 1% methanol, which means that the solution is mainly water. Higher methanol concentrations can disrupt the membrane, or cause incomplete partitioning of the ionophore into the lipophilic membrane. If you need to use more concentrated ionophore solutions then you will need to make a more concentrated stock solution (eg 10 or even 100 mM, but beware that the ionophore might not be soluble at this level) and dilute this down to the desired levels.
イオノフォアを添加
Ensure all solutions are thermally equilibrated at room temperature before starting.
Start recording with the tethaPlate fitted to the tethaPod unit, proceed for several minutes until stable baseline signals are obtained for each of the sample wells. While still recording:
- Withdraw 200 µL of the PBS buffer from the tethaPlate waste well.
- Note the time and replace with 200 µL test solution at lowest ionophore concentration.
- Record the membrane conductivity for at least 5 minutes, or until the signal plateaus.
- Withdraw 200 µL of the old test solution from the tethaPlate waste well.
- Note the time and replace with 200 µL test solution at the next higher ionophore concentration.
- Record the membrane conductivity for at least 5 minutes, or until the signal plateaus.
- Repeat steps 4 − 6, until the highest ionophore concentration is reached (or until the conductivity signal goes off scale, or the membrane ruptures)
- Withdraw 200 µL of the old test solution from the tethaPlate waste well.
- Replace with 200 µL of the PBS buffer.
- Repeat steps 8 − 9 every minute, three times, and observe whether there is a decease in conductivity, indicating that the ionophore can be 'washed out'.
測定結果
Derive conductivity ratios by dividing the observed conductivities by the baseline conductivity value, i.e. in the absence of ionophore.
Prepare a graph of log of conductivity ratio versus ionophore concentration, or log of concentration, as appropriate.
It is usual to find that there is a sudden rise in conductivity over a single decade of concentration increase. If you want better resolution on your graph then repeat the experiment using a series of test solutions prepared over this smaller concentration range.