C4D Profiler in PowerChrom Software
Contents
Introduction
This application note is obsolete. You should use the C4D Profiler V2 software to optimize C4D settings, not the C4D Profiler in PowerChrom software. See the new application note here.
This application note describes the C4D Profiler in the PowerChrom software. The software tool is designed to help select the optimum operating parameters for your buffer/background electrolyte. The software automatically records the detector’s response at every combination of frequency, amplitude and headstage gain setting, and displays the data as a series of plots. This tool also provides a useful diagnostic tool in case of problems.
Equipment Required
- ER225 C4D Data System
- The latest version of PowerChrom software
- A C4D headstage or platform, such as the ET120 C4D Headstage or ET225 Micronit Platform
- A short length of capillary, tubing or a microfluidic chip, depending on your experiment.
- The background electrolyte you intend to use for analysis.
- Deionized water.
Description
Before the C4D Profiler is used, the C4D unit should be on and connected to the computer. The C4D Profiler feature is automatically disabled when the software cannot detect a C4D unit.
The capillary should be injected with the background electrolyte you will use in subsequent experiments.
Controls
The C4D Profiler can be found in the “Windows” menu of the PowerChrom software. Its controls are shown in Figure 1 and described below:
- HG On: tick for headstage gain on.
- HG Off: tick for headstage gain off.
- When both HG On and HG Off are selected, the software will scan first with the headstage gain off, then with the headstage gain on.
- Fast Scan: this performs the test quickly by missing every second parameter.
- Start: initiates the test – the test can be stopped by choosing Stop.
- Set Bg: Set Background. When selected, this marks this run as the background.
- Show Difference: This performs a baseline subtraction using the run which has been chosen as the background. The button remains grey until a run is selected as the background and another run is displayed.
Procedure
- Turn on the C4D unit and connect to the computer.
- Inject the background electrolyte you intend to use for experiments into the capillary. Ensure the outside of the capillary is dry by wiping with a cloth. Feed the capillary into the headstage. If you are doing microchip electrophoresis, inject the background electrolyte into the chip’s channel and place the chip on the ET225 Platform.
- Don’t perform this test with no solution in the capillary as this won’t give a signal.
- Open the PowerChrom software.
- Select Manual Run.
- Click Start and enter a file name.
- Click on Stop in the Manual Sampling window.
- In the PowerChrom’s “Windows” menu, select “C4D Profiler”.
- Tick both HG On and HG Off boxes, so that both headstage gain settings are tested.
- Only select “Fast Scan” if you want to test every second voltage and frequency, which makes it four times faster. The full run takes about one minute to complete.
- In the area immediately above the graph, you should type the product serial numbers of your C4D unit and headstage, the background electrolyte you are using and its concentration.
- When ready, click the Start button in the “C4D Profiler” window.
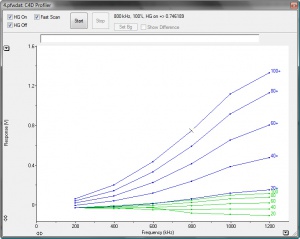
- The software will automatically plot a graph of frequency versus response, at one amplitude setting, and then repeat through the range of amplitude settings. An example of the plots is shown in Figure 2.
- The numbers on the right side of the plots are the C4D amplitude settings.
- You can change the scale the y axis, if some of the plots are off the scale.
- You can stop the scan while it is working by pressing the Stop button.
- The shaded area at the top of the graph, shows the overload region. ER225 unit will beep if overload occurs.
- If both HG On and HG Off boxes were ticked, the software will start drawing the plots with headstage gain off (green plots) and then headstage gain on (blue plots).
- The profiles from previous runs can be viewed by clicking on different the tabs in the Runs bar, normally at the bottom of the screen.
- Performing the above scan provides a good first order indication of a reasonable choice for an operating point, but there is a potentially a better method available as described below.
- First identify if headstage gain needs to be on (HG On) or off (HG On):
- If you are using a background electrolyte with low conductivity, the response for all the plots may be under 2 V. In this case, you should only use HG On.
- If you are using a background electrolyte with high conductivity, all the blue plots may go above 2V and overload the detector. In this case, you should only use HG Off.
- Perform a scan using the correct headstage gain setting.
- Dilute the background electrolyte by approximately 5% using deionized water (95% background electrolyte and 5% deionized water) and inject it into the capillary.
- Perform a scan of the diluted background electrolyte.
- Nominate the second (diluted) scan as a background file by clicking “Set Bg”. This allows the run to be used as a background run for subsequent operations.
- Return to the first run (undiluted background electrolyte) you have recorded by clicking on its tab in the Runs bar (normally at the bottom of the screen) and select Show Difference.
- The background run is now subtracted from the reference run and the resulting curves indicate where the largest signal change has occurred for the given conductivity change. The parameters in this area will be your optimum operating point.
- These C4D settings should be used for experiments when using this background electrolyte. The settings should be entered into the C4D # Amplifier window by selecting Start (in the chromatography window), Hardware Settings and C4D Amplifier.
- The recording range should be set to 50 mV (to record the small changes in conductivity which your analytes will produce) and you should click Zero before analyzing your samples.
Identifying the Optimum C4D Settings
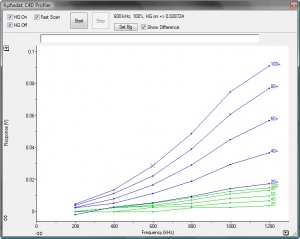
Figure 3 shows the background subtracted plots for a low conducting background electrolyte. It shows which C4D settings produced the highest output for the small change in conductivity, approximately 90mV at 100% amplitude, 1200 kHz frequency and headstage gain on.
In most experimental situations, the change of conductivity due to an analyte will be much smaller with typical signals in the 10mV range however the above test will correctly indicate the optimum C4D settings to be used.
It is generally advisable to choose parameters where the operation is still linear. In the example in Figure 3, suitable settings are 60% amplitude, 1000 kHz and with headstage gain on. In other words, find the maximum and then step back in both the frequency and amplitude to give the signal some room to move.
In some circumstances using the maximum excitation may result in the signal exceeding 2 V, at which point the detector reaches an electronic limit. In such cases a lower frequency/amplitude may be used, and in some circumstance, the headstage gain can be turned off. Cell conductivity is a proportional to the diameter and length of the measuring cell as well as the intrinsic conductivity of the buffer. This results in a very large range of conductivity values. Higher conductivities increase the current but also the frequency at which useful measurements can be made. Lower conductivities require higher excitation and higher gains.
The eDAQ headstages are configured to operate in typical capillary electrophoresis environments, but as you approach the extremes of conductivity, signals will either become too large or too small. We are prepared to modify our headstages (by special order) to adapt them to more extreme conditions.
Troubleshooting
- If you cannot see “C4D Profiler” in the PowerChrom’s “Windows” menu, then either the C4D unit is turned off, is not connected to the computer, or you are using a version of PowerChrom earlier than version 2.6.4. When no C4D device is found by the software, the C4D Profiler feature is automatically disabled.
- If you record a flat graph, with a response that stays at zero or possibly goes below zero, then your capillary is empty. You should inject your background electrolyte into the capillary.
- If you have started the scan, but don’t see any plots, try to rescale the y axis.
C4D Operation: Caution
The C4D detection method relies on the measurement of very small changes in conductivity due to analytes migrating through the detector. Unfortunately, conductivity in the detector can change for other reasons which are not always obvious. This can often causes unexpected peaks or shifts in the baseline.
NOTE: The basic C4D measurement system has negligible drift. This can be demonstrated by recording data with no capillary, or an empty capillary, in the headstage. When a change in conductivity is displayed it is can be safely assumed that it is caused by a change in conductivity of the fluid in the measurement cell.
In order to avoid or minimize these effects, the following precautions should be taken:
Temperature effects: Temperature directly affects conductivity and the changes which occur can have similar amplitudes as the peaks of interest. However temperature changes often show up as a slow step change or as a baseline drift. The C4D system itself exhibits negligible baseline drift. It is advisable therefore to allow the head stage and the capillary to stabilize at its operating temperature. This takes 20 to 30 minutes.
Pressure effects: applying hydrostatic pressure to the fluid in the capillary will cause transient baseline shifts due to subtle changes in conductivity caused by flow and associated temperature changes in the moving fluid. During measurements, time should be allowed for loading effects to stabilize.
Fluid discontinuities: Bubbles can come in different sizes and will cause excursions in conductivity. These can mimic actual analyte peaks but will, in all cases, cause a decrease in conductivity (negative peaks).
Mechanical effects: Moving the head stage or the capillary will inevitably vary the coupling between the capillary and the headstage and cause a baseline shift. External vibrations can also be coupled into the signal. It is advisable that the headstage be firmly connected to a stable mechanical support.
Electrical effects: The coupling between the headstage and the CE is dependent on very small values of capacitance whose value can be altered by external objects. For example, changing the position of the headstage can alter the baseline. External electrical noise can also couple through the headstage cable and it should always be rolled up into a minimum size and be constrained so that it does not move.
In conclusion, in order to obtain stable and repeatable results, ensure that the headstage, its connecting cables and the capillary are held firmly in place in a stable temperature, mechanical and electrical environment.