Reference Electrode Potentials
The inspiration and source for much of the information on this page was the web site Research Solutions and Sources created by the late Dr Bob Rodgers.
Do you need to convert potentials obtained with one reference electrode to the equivalent values versus another reference electrode? Then try this calculator
Two of the important general texts for reference electrodes are:
- 1. "Reference Electrodes. Theory and Practice" David J G Ives, and George J Janz, (eds) Academic Press (1961). Available at Amazon
- 2. "Handbook of Reference Electrodes". György Inzelt, Andrzej Lewenstam, and Fritz Scholz (eds). Springer-Verlag (2013). ISBN 3642361870. DOI:10.1007/978-3-642-36188-3
Contents
- 1 The Hydrogen (H2/H+) Electrode
- 2 The Silver/Silver Chloride (Ag/AgCl) Electrode
- 3 The Silver/Silver Sulfate (Ag/Ag2SO4) Electrode
- 4 The Calomel (Hg/Hg2Cl2) Electrode
- 5 The Mercury/Mercurous Sulfate (Hg/Hg2SO4) Electrode
- 6 The Mercury/Mercury Oxide (Hg/HgO) Electrode
- 7 The Copper/Copper Sulfate (Cu/CuSO4) Electrode
- 8 Other Reference Electrodes
- 9 The Salt Bridge
- 10 Organic solvents
- 11 Disclaimer
The Hydrogen (H2/H+) Electrode
The hydrogen electrode is based on the half cell equation 2H+ + 2e– 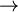 H2
H2
As can be seen from this equation, the reaction is affected by the pH (hydrogen ion concentration) of the system, and in fact a hydrogen electrode can also be used an a pH electrode!
Hydrogen electrodes are usually more fully described as the:
- 1. Standard Hydrogen Electrode (SHE), which is the basis against which other reduction/oxidation (redox) couples are reported. These days, the SHE is more of a theoretical ideal electrode. In days gone by, the electrode was known as the Normal Hydrogen Electrode {NHE) which used conditions of 1 atm pressure of H2, 25 °C, and 1 N HCl. Corrections had to be made for barometric pressure and the partial pressure of water vapour in H2 gas for the most accurate work. The SHE concept took this a step further by redefining the electrode only in terms of H+ ions without any interaction with other ions present (such as Cl–) and this means that it is not actually physically possible to construct a SHE!
- 2. Reversible Hydrogen Electrode (RHE), which usually refers to a real physical electrode that may or may not be operating under 'standard conditions'.
The hydrogen electrode is traditionally constructed by having a platinum plate or coiled wire in a vessel containing 1 N HCl. A tube through which hydrogen gas was being blown was arranged so that the gas was bubbled over the surface of the platinum. Adequate ventilation is required to avoid possible build up of hydrogen gas which is an explosion/fire hazard. These safety concerns mean that students can often pass through an entire chemistry course without ever having used, or even seen a real hydrogen electrode.
A clever way of making a modern RHE is to use a small cartridge that contains substances that produce a slow release of hydrogen gas that can be passed through a diffusion membrane containing small particles of platinum catalyst. See the Hydroflex™ electrode. This dispenses with the need to rent expensive hydrogen gas cylinders and overcomes the fire safety issues of using hydrogen gas.
The potential provided by an RHE is given by the equation
- E = E0 – (RT/nF)ln[H+]
- E = –ln(10)(RT/nF)log[H+] note that ln[H+] = ln(10) × log[H+]
- E = 2.303(RT/nF)pH note that ln(10) ≅ 2.303
- E = 0.0591pH at 25 °C and 1 atm, see Table 1. Note that E = 0.0615pH at 37 °C (body temperature, often used for biological experiments).
where:
- E is the electrode potential expressed in volts
- E0 is the standard reduction potential (zero for the hydrogen electrode)
- R is the Gas Constant, 8.314 J/mol/K
- T is the temperature, K
- n is +1 for hydrogen ion
- F is the Faraday constant, 96485 C/mol
| pH | E /V | pH | E /V |
|---|---|---|---|
| 0 | 0 | 8 | 0.473 |
| 1 | 0.059 | 9 | 0.532 |
| 2 | 0.118 | 10 | 0.591 |
| 3 | 0.177 | 11 | 0.650 |
| 4 | 0.236 | 12 | 0.709 |
| 5 | 0.296 | 13 | 0.768 |
| 6 | 0.355 | 14 | 0.827 |
| 7 | 0.414 | 15 | 0.887 |
If you are planning to use a RHE as a reference electrode directly in contact with your sample solution then they tend to work best under acidic (< pH 4) or basic conditions (> pH 10) where the sample is naturally buffered. If you are working at intermediate pH conditions then you will need to make sure that the sample has added pH buffer salts to ensure there is no appreciable change in pH during the experiment.
Often a better idea is to use the RHE with a salt bridge, or other double junction arrangement, linking to the test solution. Hydrogen around the RHE will be prevented from acting as a reductant in the test solution, and materials in the test solution will be prevented from contacting (and possibly poisoning) the platinum used in the RHE. You can also match the pH and ionic content of the solution around the RHE to be the same as the test solution in order to eliminate junction potential.
If you are using the RHE as a pH electrode then connect it to a pH meter using a Ag/AgCl, calomel, or other external reference electrode. Unlike glass pH electrodes the RHE is not affected by strongly basic solutions and can also be used in hydrofluoric acid solutions. You can calibrate the RHE in the usual manner of using two standard solutions of known pH.
The Silver/Silver Chloride (Ag/AgCl) Electrode
The Ag/AgCl electrode is by far the most popular type of laboratory reference electrode in use today.
It is constructed from a silver wire, part of which is 'chloridized' (covered with finely divided silver chloride). There are several ways to do this (see reference 7). The most popular method in industry is to dip a silver wire into molten silver chloride, while in the laboratory it is more common to electrodeposit AgCl onto a silver wire that is made the anode in an electrochemical cell containing dilute HCl (0.1 – 1.0 M) as the electrolyte solution. The chloridized end of the wire is then inserted into an electrolyte solution of KCl or NaCl.
The relevant half cell equation is:
AgCl(s) + e– 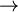 Ag(s) + Cl–
Ag(s) + Cl–
Silver chloride is quite insoluble in water (~0.2 mg/100 mL), but is slightly more soluble in concentrated chloride solutions owing to the formation of the complex ion [AgCl2]–. Though electrode potential is actually dependent on silver ion concentration (as with any metal/metal ion electrode), this is limited by the low solubility of AgCl, and thus the actual potential is effectively controlled by the chloride concentration alone.
The electrode potential is independent of hydrogen ion (acid) concentration, and so is not directly pH dependent. However when used with samples of high pH for long periods of time, hydroxide ion can contaminate electrodes with a porous tip, causing the formation of AgOH and brown/black Ag2O in place of the white/grey AgCl coating and noticeable potential drift will occur. The more basic the solution, the quicker this effect will be. Frequently replacing the filling solution of refillable Ag/AgCl electrodes can maintain the electrode, while leakless reference electrodes are largely immune to this effect.
The long term use of refillable Ag/AgCl electrodes with alkaline samples is normally avoided. Slightly basic solutions (eg pH 7-10) are often used with Ag/AgCl electrodes for extended periods. But at very basic solutions, for example 1 mol/L NaOH or more, especially at elevated temperatures, you should consider a different type of electrode.
Ag/AgCl electrodes can be used up to 100°C (depending on the materials used to make the electrode), and are commercially available from many companies. The potential does vary with temperature, but between 10 – 40°C, can be estimated by the equations (see reference 2):
- E = 205 – 0.73 × (T – 25) for an electrolyte of 3.5 M KCl
- E = 199 – 1.01 × (T – 25) for an electrolyte of saturated KCl
where T is the temperature (°C), and E is the electrode potential (mV).
| Electrolyte Solution | E vs NHE | E vs SCE | LJ | Reference |
|---|---|---|---|---|
| KCl (0.1 M) | 0.2881 | 0.047 | - | 1, 3 |
| KCl (3 M) | 0.210 | -0.032 | - | 4 |
| KCl (3.5 M) | 0.205 | -0.039 | Yes | 2 |
| KCl (sat'd) | 0.197 | -0.045 | - | 1 |
| KCl (sat'd) | 0.199 | -0.045 | Yes | 2 |
| KCl (sat'd) | 0.1988 | -0.042 | - | 2 |
| NaCl (3 M) | 0.209 | -0.035 | Yes | 5 |
| NaCl (sat'd) | 0.197 | -0.047 | Yes | 3 |
| Seawater (~0.47 M NaCl) | 0.25 | 0.01 | Yes | 6 |
Notes
- LJ, liquid junction. Value obtained using a cell which included a liquid junction potential.
- NHE, normal hydrogen electrode
- SCE, saturated calomel electrode
ET072-1 Leakless Miniature Ag/AgCl Reference Electrode
The leakless ET072 electrode enables you to perform experiments in organic solvents, hydrofluoric acids and solutions containing perchlorate or silver salts, without worrying about clogging or degradation! It can be used for long-term experiments without the concern that the filling electrolyte be run out or be diluted.
This leakless reference electrode uses a recently developed conductive junction. The filling electrolyte is confined to the tube of the electrode and won't leak at all. The junction has an extremely high conductivity with a resistance of under 10 kilo ohms. The electrode is robust with high mechanical stability, will not swell and is resistant to organic solvents. The junction potential is independent of the sample or of its ionic strength. The body of the electrode is made from polyether ether ketone (PEEK) which gives it it’s high chemical resistance. The filling electrolyte (3.4 molar KCl) doesn’t leak into your sample; this prevents contamination of your sample with ions such as chloride or potassium. There is no clogging and no requirement for a double junction. The electrode doesn’t contain any glass so it can be used in solution with hydrofluoric acid.
References
- 1. "Electrochemical Methods: Fundamentals and Applications", A J Bard and L R Faulkner, John Wiley & Sons, NY (2000). ISBN 0471405213. See the table on inside back cover.
- 2. "Electrochemistry for Chemists, Second Edition", D T Sawyer, A J Sobkowiak, J Roberts, Jr., John Wiley & Sons, NY (1995). ISBN 0471594687. See Table 5.3
- 3. "Handbook of Analytical Chemistry", L Meites (ed.), McGraw Hill, NY (1963). ISBN 0070413363. See Section 5.
- 4. E P Friis, J E T Anderson, L L Madsen, N Bonander, Per Moller, J Ulstrup, Electrochimica Acta, 43, 1114-1122, 1998. DOI: 10.1016/S0013-4686(98)99006-5
- 7. "Reference Electrodes. Theory and Practice" David J G Ives, and George J Janz, (eds) Academic Press (1961). See pages 203 – 213.
- 8. Standard Potential of the Silver-Silver Chloride Electrode. R G Bates and J B MacAskill, Pure & Applied Chemistry, 50, 1701—1706, 1978. IUPAC
- 9. An inexpensive, commercial-type, reference electrode. T H Randle and P J Kelly, Journal of Chemical Education, 61, 721, 1984. DOI: 10.1021/ed061p721
- 10. Easy-to-Make Ag/AgCl Reference Electrode. Gaston A. East , M. A. del Valle, Journal of Chemical Education, 77, 97, 2000. DOI: 10.1021/ed077p97
The Silver/Silver Sulfate (Ag/Ag2SO4) Electrode
The Ag/Ag2SO4 electrode is enjoying increasing popularity as a replacement for the Hg/HgSO4 electrode, and where a mercury– and chloride–free electrode is required. It is especially useful in experiments to do with lead–acid batteries (reference 8).
It is constructed from a silver wire, part of which is covered with finely divided silver sulfate, usually prepared electrochemically using the silver wire as an anode in a sulfuric acid electrolyte solution, see reference 7. If making your own electrode be careful to exclude even trace amounts of halide in the starting reagents as silver halide salts around the silver wire will affect the reference potential.
The relevant half cell equation is: Ag2SO4(s) + 2e– 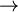 2Ag(s) + SO42–
2Ag(s) + SO42–
Silver sulfate is sparingly soluble in water (~0.83 g/100 mL). Thus the electrolyte must be high in sulfate ion concentration to ensure that it is saturated in silver ion.
Commercial electrodes are available from:
- Koslow Scientific (USA)
| Electrolyte Solution | E vs NHE | E vs SCE | LJ | Reference |
|---|---|---|---|---|
| H2SO4 (0.5 M) | 0.72 | 0.48 | - | 1, 3, 5 |
| H2SO4 (1.0 M) | 0.71 | 0.47 | Yes | 4, 5 |
| K2SO4 (sat'd) | 0.68 | 0.44 | - | 2, 5 |
Notes
- LJ, liquid junction. Value obtained using a cell which included a liquid junction potential.
- NHE, normal hydrogen electrode
- SCE, saturated calomel electrode
- See more information about how these numbers were calculated.
Reference 7 suggests that the electrolyte filling solution should have sulfate concentrations higher than 0.01 M and silver ion concentrations between 0.001 – 1 mM, for best performance.
References
- 1. "Electrochemical Methods: Fundamentals and Applications", A J Bard and L R Faulkner, John Wiley & Sons, NY (2000). ISBN 0471405213. See the table on inside back cover.
- 2. "Electrochemistry for Chemists, Second Edition", D T Sawyer, A J Sobkowiak, J Roberts, Jr., John Wiley & Sons, NY (1995). ISBN 0471594687. See Table 5.3
- 3. "Handbook of Analytical Chemistry", L Meites (ed.), McGraw Hill, NY (1963). ISBN 0070413363. See Section 5.
- 4. Electrochemical Deposition of Co under the Influence of High Magnetic Fields, M. Uhlemann, A. Krause, J. P. Chopart, and A. Gebert, Journal of the Electrochemical Society, 152, C817-C826, 2005. DOI: 10.1149/1.2073167
- 5. Calculated by adding 40 mV to the corresponding mercury-mercurous sulfate electrode, see the section on these electrodes, below.
- 7. On the stability of the silver/silver sulfate reference electrode, Matěj Velický, Kin Y. Tamb, and Robert A. W. Dryfe, Analytical Methods, 4, 1207-1211, 2012.
- 8. Silver–silver sulfate reference electrodes for lead-acid batteries, Paul Ruetschi, Journal of Power Sources, 113, 363–370, 2003. DOI: 10.1016/S0378-7753(02)00549-9
The Calomel (Hg/Hg2Cl2) Electrode
WARNING. Mercury and its salts are highly toxic. Only suitably qualified persons should attempt to make mercury containing electrodes.
The calomel electrode (more accurately described as the 'mercury/calomel electrode') is usually constructed from a platinum wire inserted into a mixture of calomel (mercurous chloride, Hg2Cl2) and liquid mercury, with an electrolyte solution of KCl or NaCl.
Calomel is insoluble in water (~0.4 mg/100 mL) to about the same extent as silver chloride, see reference 5.
The relevant half cell equation is:
Hg2Cl2(s) + 2e– 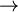 2Hg(liq) + 2Cl–
2Hg(liq) + 2Cl–
As this equation implies, the electrode potential is dependent on chloride concentration, but independent of hydrogen ion (acid) concentration.
Calomel electrodes are unstable much above 50°C owing to the disproportionation reaction: Hg2Cl2 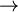 Hg(liq) + HgCl2
Hg(liq) + HgCl2
Commercial calomel electrodes are available from:
- Koslow Scientific (USA)
- ALS Co. Ltd (Japan)
- Ionode Pty Ltd (Australia)
and some other companies that make pH electrodes (though many such companies have stopped production because of falling demand). In Europe the use of calomel electrodes is increasingly problematic because many countries no longer permit the use of mercury-containing devices.
| Electrolyte Solution | E vs NHE | E vs SCE | LJ | Reference |
|---|---|---|---|---|
| KCl (0.1 M) | 0.3337 | 0.0925 | - | 1, 3 |
| KCl (0.1 M) | 0.336 | 0.092 | Yes | 2 |
| NCE, KCl (1 M) | 0.2801 | 0.0389 | - | 1, 3 |
| NCE, KCl (1 M) | 0.283 | 0.039 | Yes | 2 |
| KCl (3.5M) | 0.250 | 0.006 | Yes | 2 |
| SCE, KCl (sat'd) | 0.2412 | 0 | - | 1, 3 |
| SCE, KCl (sat'd) | 0.244 | 0 | Yes | 2 |
| SSCE, NaCl (sat'd) | 0.2360 | -0.0052 | - | 1 |
Notes
- LJ, liquid junction. Value obtained using a cell which included a liquid junction potential.
- NCE, normal calomel electrode
- NHE, normal hydrogen electrode
- SCE, saturated calomel electrode
- SSCE, saturated salt calomel electrode
- For values at other temperatures use this calculator.
References
- 1. "Electrochemical Methods: Fundamentals and Applications", A J Bard and L R Faulkner, John Wiley & Sons, NY (2000). ISBN 0471405213. See the table on inside back cover.
- 2. "Electrochemistry for Chemists, Second Edition", D T Sawyer, A J Sobkowiak, J Roberts, Jr., John Wiley & Sons, NY (1995). ISBN 0471594687. See Section 5.2.
- 3. "Handbook of Analytical Chemistry", L Meites (ed.), McGraw Hill, NY (1963). ISBN 0070413363. See Section 5.
- 4. Standard E.m.f. of the hydrogen-calomel cell from 0 to 45°C. S R Gupta, G J Hills and D J G Ives. Transactions of the Faraday Society, 59, 1874-1885, 1963. DOI: 10.1039/TF9635901874
- 5. Compilation and Evaluation of Solubility Data in the Mercury(I) Chloride–Water System. Y Marcus, Journal of Physical Chemistry Reference Data, 9, 1307–1329, 1980. www.nist.gov/data/PDFfiles/jpcrd173.pdf
- 6. A versatile compact reference electrode. Kousuke Kusuda, J.]ournal of Chemical Education, 66, 531, 1989. DOI: 10.1021/ed066p531
The Mercury/Mercurous Sulfate (Hg/Hg2SO4) Electrode
WARNING. Mercury and its salts are highly toxic. Only suitably qualified persons should attempt to make mercury containing electrodes.
The Hg/Hg2SO4 electrode is used where a chloride free electrode is required. It is especially used in experiments to do with lead–acid batteries (reference 8).
The electrode is usually constructed from a platinum wire inserted into a mixture of Hg2SO4 and liquid mercury, with an electrolyte solution of K2SO4 or H2SO4.
The relevant half cell equation is: Hg2SO4(s) + 2e– 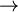 2Hg(liq) + SO42–
2Hg(liq) + SO42–
Mercurous sulfate is slightly soluble in water (51 mg/100 mL).
Commercial electrodes are available from:
- Koslow Scientific (USA)
- ALS Co. Ltd (Japan)
| Electrolyte Solution | E vs NHE | E vs SCE | LJ | Reference |
|---|---|---|---|---|
| H2SO4 (0.5 M) | 0.68 | 0.44 | - | 1 |
| H2SO4 (0.5 M) | 0.682 | 0.441 | - | 3 |
| H2SO4 (1.0 M) | 0.674 | 0.430 | Yes | 2 |
| K2SO4 (sat'd) | 0.64 | 0.40 | - | 1 |
| K2SO4 (sat'd) | 0.65 | 0.41 | - | 3 |
Notes
- LJ, liquid junction. Value obtained using a cell which included a liquid junction potential.
- NHE, normal hydrogen electrode
- SCE, saturated calomel electrode
References
- 1. "Electrochemical Methods: Fundamentals and Applications", A J Bard and L R Faulkner, John Wiley & Sons, NY (2000). ISBN 0471405213. See the table on inside back cover.
- 2. M Uhlemann, A Krause, JP Chopart, A Gebert, J. Electrochem. Soc., 152 (2005), C817-C826.
- 3. "Handbook of Analytical Chemistry", L Meites (ed.), McGraw Hill, NY (1963). ISBN 0070413363. See Section 5.
The Mercury/Mercury Oxide (Hg/HgO) Electrode
WARNING. Mercury and its salts are highly toxic. Only suitably qualified persons should attempt to make mercury containing electrodes.
Many conventional reference electrodes (Ag/AgCl, Ag/Ag2SO4, calomel) will have limited life times in very alkaline solutions. This is caused by hydroxide diffusing into the electrode filling solution, and forming silver hydroxy/oxide species on the silver wire (which becomes brown/blackened) and which then causes a shift in the electrode potential. This can be ameliorated, to some extent, by the use of a double junction to slow the hydroxide diffusion, but an electrode that is stable under alkaline conditions is often a better answer.
The Hg/HgO electrode is ideal for use in alkaline solutions. The relevant half cell equation is:
HgO(s) + 2e– + H2O 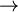 Hg(liq) + 2OH–
Hg(liq) + 2OH–
As the half cell equation suggests the potential is dependent on the hydroxide ion concentration used in the electrolyte solution. It is usual to match the hydroxide concentration of the filling solution to that of the sample to minimise the junction potential.
Mercuric oxide is sparingly soluble in water (~5.3 mg/100 mL), and alkaline solutions.
A commercial electrode is available (see reference 2). If you are attempting to make your own electrode then you should be aware of the information in reference 3. A simple procedure for making an electrode is given in reference 4, although you may want to try substituting carbon fiber for the toxic asbestos fibre.
| Electrolyte Solution | E vs NHE | E vs SCE | LJ | Reference |
|---|---|---|---|---|
| NaOH (0.1 M) | 0.165 | -0.076 | - | 1 |
| NaOH (1 M) | 0.140 | -0.101 | - | 1 |
| KOH (20%) | 0.098 | - | ? | 2 |
Notes
- LJ, liquid junction. Value obtained using a cell which included a liquid junction potential.
- NHE, normal hydrogen electrode
- SCE, saturated calomel electrode
References
- 1. "Handbook of Analytical Chemistry", L Meites (ed.), McGraw Hill, NY (1963). ISBN 0070413363. See Section 5.
- 4. A simple procedure for making Hg\HgO reference electrodes. Raymond Thacker, Journal of Chemical Education, 45, 180, 1968. DOI: 10.1021/ed045p180
- 5. Behaviour of the mercury, mercuric oxide electrode in alkaline solutions in the temperature range 298–363 K. H Corti, R Fernández-Prini, Journal of the Chemical Society, Faraday Transactions 1, 78, 545-554, 1982. DOI: 10.1039/F19827800545
The Copper/Copper Sulfate (Cu/CuSO4) Electrode
The saturated (Cu/CuSO4) electrode is often used as a reference in the field for commercial applications of cathodic protection.
The relevant half cell equation is: Cu2+ + 2e– 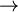 Cu(s)
Cu(s)
As the half cell equation suggests the potential is independent of acid concentration. However basic samples should be avoided as hydroxide leaching into the electrolyte compartment will cause formation of insoluble copper hydroxide and copper oxide.
Many commercial electrodes are available, for example:
- • Tinker & Rasor
- • MC Miller
- • Matcor
If you are attempting to make your own electrode then you need to saturate the solution with copper sulfate and have excess crystalline material in the electrolyte compartment. See the Corrosion Doctors' website for a recipe.
The potential of the Cu/CuSO4 electrode is +0.314 V with respect to the standard hydrogen electrode (SHE).
.
Other Reference Electrodes
It is possible to construct many other types of reference electrodes, many of which are used to cope with special conditions peculiar to the experiment being undertaken, for example see references 1 - 3. For teaching, a Cu+/Cu2+ has being described, reference 4, while for alkaline conditions an electrode using manganese dioxide has been proposed, reference 5.
References
- 1. "Reference Electrodes. Theory and Practice" David J G Ives, and George J Janz, (eds) Academic Press (1961).
- 2. "Handbook of Reference Electrodes". György Inzelt, Andrzej Lewenstam, and Fritz Scholz (eds). Springer-Verlag (2013). ISBN 3642361870. DOI:10.1007/978-3-642-36188-3
- 3. Preparation and characterization of palladium-hydride-coated titanium as a reference electrode for the supercritical carbon dioxide emulsion electrochemical system. Yung-Yi Wua, Chun-Tsung Hsua, Tso-Fu Mark Changb, Masato Soneb, Chi-Chang Hu, Electrochimica Acta 155, 209–216, 2015. DOI: 10.1016/j.electacta.2014.12.141
- 4. Low-Cost Reference Electrode for Potentiotitrimetry. P. Riyazuddin, Journal of Chemical Education, 71, 167, 1994. DOI: 10.1021/ed071p167
- 5. Assembly of a Robust and Economical MnO2-Based Reference Electrode. Robert C. Massé and James B. Gerken, Journal of Chemical Education, 92, 110–115, 2015 DOI: 10.1021/ed500587a
The Salt Bridge
These reference electrodes, above, all need a salt bridge which is a junction or portal that connects the internal electrolyte solution of the electrode to the external sample solution. To limit the diffusion of ions across the junction (which would contaminate both the internal and external solution with foreign ions) some semipermeable material is usually inserted into the portal. This is often a plug of mineral fibers (asbestos was often used in the past until its toxicity was discovered), or porous ceramic, glass, or plastic. Obviously this plug is selected to be as chemically inert as possible. In some cases the junction is left completely open (just a pinhole opening is used) or the electrode is constructed so that a sleeve of electrolyte solution is constructed between two close fitting pieces of glass. If the plug is too impermeable then it increases the electrical resistance between the reference electrode and the sample, and if too permeable it allows too much exchange of the ionic materials. There is always a trade off between these two extremes. Electrode manufacturers will have optimised permeability for what they perceive to be applications for which the electrode is designed but occasionally their choice of material may became a source of problems, for example see reference 2. Similarly, if you want to make your own reference electrode then do not neglect to consider the choice of material for the junction.
References
- 1. "Reference Electrodes. Theory and Practice" David J G Ives, and George J Janz, (eds) Academic Press (1961).
- 2. Reference Electrodes with Salt Bridges Contained in Nanoporous Glass: An Underappreciated Source of Error. Maral P. S. Mousavi and Philippe Bühlmann, Analytical Chemistry, 85, 8895−8901, 2013. DOI: 10.1021/ac402170u
Organic solvents
If your experiments require the use of an organic solvent the use of classical aqueous reference electrode may be impracticable.
Cyclic voltammetry of organics, organometallics, or of complex ions, often requires the use of organic solvents such as dichloromethane, acetonitrile, tetrahydrofuran, propylene carbonate, or dimethylsulfoxide. In these cases the International Union of Pure and Applied Chemistry (IUPAC) recommends the use of the ferrocenium ion/ferrocene or bis(biphenyl)chromium(I)/bis(biphenyl)chromium(0) couple, reference 1. However a wider range of materials are considered in reference 2.
The use of the ferrocene/ferrocenium ion (Fc/Fc+) couple has also been studied as to providing an estimate for the uncompensated resistance encountered when doing cyclic voltammetry in organic solvents, reference 3.
References
- 1. Recommendations on reporting electrode potentials in nonaqueous solvents. G Gritzner, J Kuta, Pure and Applied Chemistry 56, 461-466, 1984. DOI 10.1351/pac198456040461
- 2. Reference Redox Systems in Nonaqueous Systems and the Relation of Electrode Potentials in Nonaqueous and Mixed Solvents to Standard Potentials in Water. Gerhard Gritzner, Chapter 2 in "Handbook of Reference Electrodes". György Inzelt, Andrzej Lewenstam, and Fritz Scholz (eds). Springer-Verlag (2013). ISBN 3642361870. DOI:10.1007/978-3-642-36188-3
- 3. Use of the Ferrocene Oxidation Process To Provide Both Reference Electrode Potential Calibration and a Simple Measurement (via Semiintegration) of the Uncompensated Resistance in Cyclic Voltammetric Studies in High-Resistance Organic Solvents. Alan M. Bond, Keith B. Oldham, and Graeme A. Snook, Analytical Chemistry, 72, 3492-3496, 2000. DOI: 10.1021/ac000020j
Disclaimer
Any products mentioned on this page are for informational purposes only and constitute neither an endorsement nor a recommendation.