Difference between revisions of "Frequently Asked Questions Electrodes"
(→ET073 Reference Electrode in Organic solvent) |
|||
| (50 intermediate revisions by 4 users not shown) | |||
| Line 2: | Line 2: | ||
__TOC__ | __TOC__ | ||
| − | |||
| + | = ET069 and ET072 Leakless Ag/AgCl Reference Electrodes = | ||
| − | + | == General == | |
| − | + | === General Notes on Use === | |
| − | 1. | + | Leakless electrodes [https://www.edaq.com/ET069-1 ET069] and [https://www.edaq.com/ET072-1 ET072] |
| + | * DO NOT APPLY TOO MUCH PRESSURE ON THE CONNECTOR PIN. | ||
| + | * Remove the cap protecting the tip before use by gripping the electrode by its PEEK body and carefully removing the plastic cap. | ||
| − | + | The electrode utilizes a unique junction which is robust and highly conductive but not porous. There is no glass used in the construction. | |
| − | + | The electrode cannot be refilled. | |
| − | + | The electrode is not affected by hydrofluoric acid and common dilute acids, and bases. It is resistant to most commonly used organic solvents. If the electrode is left to dry for a very long period of time, it should be immersed in deiniozed water for a few hours before use. | |
| − | + | Read instructions regarding Long Term storage of electrodes. | |
| − | + | If using the electrode in solutions containing ions that form precipitates with chloride and/or potassium ions, then DO NOT store the electrode in potassium chloride solution. | |
| − | + | If using the electrode in dry organic solvent, the electrode should be rinsed with acteone (to remove water), then rinsed with the final solvent. The electrode should be stored in 0.05-0.1 M sulfuric acid, NOT the organic solvent when not in use. | |
| − | + | Depending on the choice of solvent, substrate molecules, and level of care, the electrode should last many months if not several years. | |
| − | + | An old electrode suffering from potential drift can sometimes be reactivated by subjecting it to a large oxidizing potential (+4 V) in a two electrode system (use a wire for the counter electrode) in a KCl solution for 10 –15 seconds then waiting 30 seconds for stabilization. | |
| + | Material adsorbed on the electrode surface can be removed by careful polishing on fine sand paper (or with abrasive powder). Alternatively, try immersing in strong acid (e.g. 6 mol/L H2SO4) for 30 minutes then sonicate, and repeat if necessary. | ||
| − | + | Download the pdf Instruction Sheet from: [[File:ET069 ET072 Instruction Sheet.pdf]] | |
| − | |||
| − | + | === General Questions === | |
| + | > 1. what is the resistance of the leakless ref electrode ? | ||
| − | + | less than 10 kohm | |
| − | + | > 2. is there a difference between the miniature and the larger LF electrodes (aside from the size) ? | |
| − | + | No. | |
| − | + | ||
| + | > 3. is there any experience with use of these LF electrodes used over months or even years (drift of potential due to ions intruding from the electrolyte?) | ||
| − | + | Depending on conditions (solvents, temperature, etc) and frequency of use you can usually expect months to years of use. | |
| − | + | > 4. what is the pin material ? | |
| − | + | gold plated | |
| − | + | > 5. What is the maximum operating temperature. | |
| − | + | The electrode will operate at temperatures below 90 Centigrade. Exceeding this temperature can cause boiling of the internal solution leading potentially to electrode damage. | |
| − | + | > 6. '''Electrodes showing drift''' | |
| + | Customer reported drift of 50mV after a week of use in a fixed 7.5pH environment. | ||
| − | + | ANSWER: The electrode can handle extreme cases of acid and bases. However there might be some material aadsorbed on the electrode surface. Please dip in 0.5-1M sulfuric acid and/or 1M hydroxide for a few minutes and then acetone or ethanol for additional cleaning. | |
| + | If the electrode has been left dry for some time, soak for a few hours in water. | ||
| − | + | > 7. The ET072 electrode is characterized by its leakless property. The application note says that the filling electrolyte (3.4 M KCl) doesn’t leak into the sample. Without ion exchange, how does the charge transfer achieve? | |
| − | + | ANSWER: The junction was formulated to provide conductivity without the need to leak potassium and chloride ions. | |
| − | + | > 8. Would there be any problem if ET072 is used in electrolytes with low ion concentration? One of my experiments use DI water as the electrolyte. | |
| − | + | ANSWER: No problem. you can use it in pure water. | |
| − | + | > 9. Will the ET072/ET069 electrode be OK for use in the organic solvent tributyl phosphate (TBP)? I would like to use it in TBP that also will probably have nitric acid (HNO3) in it in 0.5 M to 3 M concentration. There will be some water in this; it will not be dry. Will the electrode be OK in this system? | |
| − | + | ANSWER: Yes, it can be used in (TBP). The electrodes are resistant to nitric acid. We tried higher concentrations and did not damage them. | |
| − | + | > 10. Recently we purchased some small custom electrodes that were packed with a small vial to ensure electrodes don't dry out during shipping. | |
| − | + | Customer is asking "What is the liquid ? | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ANSWER: It is diluted, about 0.05 M sulfuric acid with little potassium chloride. Concentrations are not crucial. | |
| + | Frequent users of the 1 mm OD, claim they perform better when shipped in vials. Some times we ship the LF-1.6 in vials. | ||
| − | + | > 11. I am currently using your ET072 leakless Ag/AgCl reference electrode and it works really nice. However, the temperature range is stated between 0-90 C, we are planning to do tests with temperatures up to 140 C, do you have any reference electrodes with the same dimensions as the ET072 that handles temperatures that high? | |
| − | + | ANSWER: If the user going to use the electrode over short periods of time, say 30 min each time, the junction and PEEK body can handle that. The only worry is the boiling of the filling solutionmay cause damage the connector seal. The user can immerse just 5 to 10 mm of the tip in the heated solution if possible. Ask the user to try it. If damaged we can replace and think of a different method of preparation such as leaving little room inside the electrode for expansion. Remember that we can make very long electrodes if the solution container is deep or make electrodes bent at certain angles. | |
| − | + | > 12. Your website and documentation is unclear on what the storage solution for this electrode should be. In one place, it says "aqueous solution" while in another place it says "distilled water" and in a final place it says "0.05 to 0.1 M sulfuric acid". Could you clarify which storage solution I should use for my application? | |
| − | + | ANSWER: They can be stored in distilled water if needed, or the diluted sulfuric acid. Indeed, any aqueous solution should be fine. Just the 0.05 M sulfuric is recommended but NOT a must. | |
| − | + | >13. The ET072 electrode is characterized by its leakless property. The application note says that the filling electrolyte (3.4 M KCl) doesn’t leak into the sample. Without ion exchange, how does the charge transfer achieve? | |
| − | + | ANSWER: The junction was formulated to provide conductivity without the need to leak potassium and chloride ions. | |
| − | + | >14. Would there be any problem if ET072 is used in electrolytes with low ion concentration? One of my experiments use DI water as the electrolyte. If the ET072 is used as the reference electrode in such experiments, is it possible to estimate its equilibrium potential through calculation? | |
| − | + | ANSWER: No problem. you can use it in pure water. This Ag/AgCl/KCl electrode is about 224 mV vs standard hydrogen electrode. | |
| − | + | >15. I would like to ask you about the potential of reference electrode vs. SHE. | |
| − | + | ANSWER: It is about 224 mV vs SHE. | |
| − | === | + | >16. Do you have any information about the compatibility of the AMANI1600 Micro pH |
| + | sensor with a Mettler Toledo pH meter, which uses a sensor like this: | ||
| + | https://www.mt.com/gb/en/home/products/Laboratory_Analytics_Browse/pH-meter/sensor/pH-sensor/InLab-Routine.html | ||
| + | |||
| + | ANSWER: Our pH sensors come with a BNC connector. So they compatible with any pH meter or potentiometer that accepts a BNC connection. | ||
| + | What is the connection on the meter, not the sensor, because the sensor in the link screws to another end or socket which connects it to meter. | ||
| + | |||
| + | >17. For my research I have to build my CV cells in the glovebox and therefore vacuum is applied on my ET072-1 electrodes. Thus, I want to know how vacuum resistant these electrodes are or if they get broken during vacuum application? | ||
| + | |||
| + | ANSWER: The electrode should be fine in vacuum. The ET-72 ( LF-2) has a thick wall and is completely sealed. The user should try it and if the electrode damaged "explodes" we can replace or refund it. I am sure over the years researchers have used ET072 under such conditions and I did not hear a complaint. | ||
| + | |||
| + | >18. Can you please advise me if ET072/ET069 can be used with Toluene and dibenzyltoluen ? | ||
| + | |||
| + | ANSWER: Yes, it can be used. I do not expect any problem. | ||
| + | |||
| + | == Storage == | ||
| + | |||
| + | === Long term storage === | ||
| + | |||
| + | Leakless electrodes ET069 and ET072 can be stored for both short and long term duration's in distilled water, or the diluted sulfuric acid, 0.05 to 0.1 M sulfuric acid. Indeed, any aqueous solution should be fine. Just the 0.05 M sulfuric is recommended but NOT a must. | ||
| + | Based on this storage method the electrodes should last for many months if not several years. Because eDAQ has no control over the use of these electrodes our warranty is limited to 3 months from date of invoice. | ||
| + | |||
| + | Please remember these electrodes are designed primarily for ease of use, and generally need to be replaced when they begin to show excessive drift. See information sheet at | ||
| + | |||
| + | https://www.edaq.com/product_sheets/transducers/ET072_Leakless_Miniature_Ag-AgCl_Reference_Electrode.pdf. for Maintenance details. | ||
| + | |||
| + | == Use and Solvents == | ||
| + | |||
| + | === Effect of high pH values === | ||
QUESTION | QUESTION | ||
| − | + | A customer would like to know if the [https://www.edaq.com/ET072-1 ET072] Leakless Miniature Ag/AgCl Reference Electrode is stable in extreme pH-conditions. It would be used for several weeks in pH 12-13 environments. Would that be a problem you think ? | |
ANSWER | ANSWER | ||
| − | + | The LF electrodes were kept in 5 M potassium hydroxide for few days and in 3 M in sodium hydroxide for over a year. No junction damage occurred. A little shift in potential might occur, but the electrode functions well | |
| − | + | ||
| − | === | + | === Extreme operating conditions === |
QUESTION | QUESTION | ||
| − | + | # A customer would like to know if the ET072 Leakless Miniature Ag/AgCl Reference Electrode will endure 1M KOH solution @ 80°C conditions ? | |
| − | + | ||
| − | + | # Is your ET074 glassy carbon electrodes heat-resistant and work up to 140 degrees Celsius? | |
ANSWER | ANSWER | ||
| − | + | # ET072 was boiled in KOH for 15 min then left it to cool down for one hour in KOH. There were no apparent change in conductivity or potential. | |
| + | Soaked one ET072 in 5 M KOH at room temperature for weeks. There was no damage. | ||
| − | + | This is an important advantage, since researchers use toxic mercury/mercury oxide electrodes with porous junctions because normal Ag/AgCl electrodes are not stable due to the formation of Ag(OH) which is converted to Ag2O. So our ET072 electrode can be used in extreme acid or extreme base. These electrodes have been soaked for long periods >300days and even boiled in 100g/l Sulfuric acid without damage. | |
| − | + | # ET074 Glassy Carbon voltammetric disk electrode, will operate at temperatures below 90 Centigrade. Exceeding this temperature can cause electrode damage. | |
| − | + | ||
| − | + | ||
| − | + | === High resistance of ET072 === | |
| − | + | QUESTION | |
| + | I recently purchased a miniature leakless Ag/AgCl reference electrode from eDAQ, and I am trying to use it for cyclic voltammetry in a rotating disk electrode setup. I am getting very bizarre results and my potentiostat is having trouble giving me a quality uncompensated resistance value for the cell (gives high phase error). I did not have this problem when working with an Ag/AgCl with a porous junction. Is the resistance of these leakless electrodes necessarily higher than that of those with porous frits? Is there a difference in experimental applications between your miniature leakless electrodes and regular-sized ones (i.e., is there a range of suitable currents for the smaller electrode vs. the larger one)? | ||
| − | === Use | + | ANSWER |
| + | |||
| + | The ET072 has an internal resistance (impedance) of less than 10 kohm. In most cases this will not present a problem for the potentiostat however in some cases large electrode impedance (depending on factors such as choice of electrolyte solution, distances between working, reference, and auxiliary electrodes, etc) may cause potentiostat instability, especially if positive feedback iR compensation is being used. | ||
| + | |||
| + | In such cases it may be necessary to: | ||
| + | |||
| + | 1. run the potentiostat in 'high stability' mode (refer to the potentiostat manual) | ||
| + | |||
| + | 2. select a different reference electrode with lower impedance. The 'leakier' the electrode the less resistance/impedance it will have. | ||
| + | |||
| + | 3. change the electrochemical cell design and especially bring the electrodes closer together, | ||
| + | |||
| + | 4. increase the concentration of the background electrolyte, or | ||
| + | |||
| + | 5. introduce a capacitor of appropriate size between the reference and auxiliary electrode. | ||
| + | |||
| + | |||
| + | === Use in Ionic Liquids === | ||
QUESTION | QUESTION | ||
| Line 155: | Line 192: | ||
| − | + | === Use in aqueous solutions of Bases and Acids === | |
| − | === Use | + | |
QUESTION | QUESTION | ||
| − | Is it possible to use | + | Is it possible to use ET072 and ET069 electrodes in diluted HCLO4 (aqueous solution at ~2M) during several hours? |
Is it possible to use it in diluted HF solutions (aqueous solution up to 5M) ? - within which pH range (aqueous solution) can it be used? | Is it possible to use it in diluted HF solutions (aqueous solution up to 5M) ? - within which pH range (aqueous solution) can it be used? | ||
| Line 167: | Line 203: | ||
The electrode material is not affected by the acids mentioned above. This was established years ago. The electrodes can handle 5M acid or 5M base. There might be a small shift in potential which is reversible but no physical damage or leakage occurs. Can be used over the full range of pH and temperatures | The electrode material is not affected by the acids mentioned above. This was established years ago. The electrodes can handle 5M acid or 5M base. There might be a small shift in potential which is reversible but no physical damage or leakage occurs. Can be used over the full range of pH and temperatures | ||
| − | |||
| − | + | === Use in aggressive solvents === | |
| − | === Use | + | |
QUESTION | QUESTION | ||
| − | Can the | + | Can the ET072 and ET069 leakless electrodes be used in organic solvents, perchlorate and silver salts solutions, Dimethylformamide or Hydrofluoric acids? |
| − | salts solutions, or Hydrofluoric acids? | + | |
ANSWER | ANSWER | ||
| Line 181: | Line 214: | ||
Our leakless electrodes ET072 and ET069 enable you to | Our leakless electrodes ET072 and ET069 enable you to | ||
perform your experiments in organic solvents, perchlorate and silver | perform your experiments in organic solvents, perchlorate and silver | ||
| − | salts solutions, or Hydrofluoric acids without being worried about | + | salts solutions, Dimethylformamide or Hydrofluoric acids without being worried about |
clogging or degradation! | clogging or degradation! | ||
It can also be used for long term experiments without the worry that | It can also be used for long term experiments without the worry that | ||
| Line 198: | Line 231: | ||
not involve any glass, it can be used in hydrofluoric acid solutions. | not involve any glass, it can be used in hydrofluoric acid solutions. | ||
| − | === Use of | + | |
| + | = Other Electrodes = | ||
| + | |||
| + | |||
| + | === When do Electrodes require cleaning? === | ||
| + | |||
| + | |||
| + | See the application note [[Cleaning and Polishing Voltammetric Electrodes]] | ||
| + | |||
| + | ANSWER | ||
| + | |||
| + | 1. If they look dirty they probably are and cleaning should be done. | ||
| + | |||
| + | 2. If the electrochemistry reaction produces any sort of insoluble material (including any sort of electrodeposition or electropolymerization reaction) then cleaning of the working electrode (and maybe also the auxiliary electrode) will be required. | ||
| + | |||
| + | 3. If you get strange peaks in a cyclic voltammogram run when the the electrodes are placed in fresh solvent/electrolyte then (assuming the electrolyte solution is pure) then the working electrode surface should be cleaned. | ||
| + | |||
| + | 4. If the current is unexpectedly small then the working (or auxiliary) electrode surface may be coated with a non conductive material. Although by this stage it would normally be visibly fouled. | ||
| + | |||
| + | 5. If you get the expected voltammetric peaks but at wrong E values then the reference electrode may be exhausted and need regenerating or replacement. | ||
| + | |||
| + | 6. If you get oscillations/noise in starting a volumetric experiment then the reference electrode may be clogged or broken giving an open circuit. If this is suspected then repeat the experiment without the reference electrode attached. If you get a similar result then the reference electrode needs cleaning or replacement. | ||
| + | |||
| + | Our [https://www.edaq.com/ET030 ET030 Electrode Polishing Kit] is useful for cleaning electrodes. | ||
| + | |||
| + | |||
| + | === ET073 Use in Organic Solvents === | ||
| + | |||
| + | '''Question:''' I want to use the eDAQ [https://www.edaq.com/ET073-1 ET073] Refillable Miniature Ag/AgCl Reference Electrode in water-free conditions. Therefore, I filled it with AgNO3 (0.1M) in ACN. However, the potential of this Ag/AgNO3 reference electrode is not constant. Should I remove the darker AgCl coating from the silver wire? | ||
| + | |||
| + | '''Answer provided by Dr Paul Duckworth''':Customer is attempting to make a silver/silver ion electrode for use in organic solvents (in the case 'AN' acetonitrile). | ||
| + | |||
| + | To do this the AgCl coating on the silver wire MUST be completely removed. This can be done by using abrasive paper to rub the AgCl coating off. You can also use 1 mol/L ammonia solution to dissolve the AgCl. | ||
| + | |||
| + | You can then fill the ET073 electrode with 0.1 mol/L silver salt solution (usually silver nitrate, tetrafluroborate, or hexafluorophosphate). | ||
| + | |||
| + | You should now get a steady potential (but you need to keep temperature constant, to at least within 1 centigrade degree, to keep the potential constant to within 1 mV). | ||
| + | |||
| + | Note that this type of reference electrode must not be used in a solution that contains ions like Cl-, Br-, I-, SCN-, OH-, S2-, or any other ion that will react with Ag+ ion to form a precipitate. | ||
| + | |||
| + | |||
| + | === Measuring Sugars using Zensor Electrodes === | ||
QUESTION | QUESTION | ||
| − | + | Customer asked about measuring total carbohydrates (Sugars in sweet beverages) | |
ANSWER | ANSWER | ||
| − | Hydroflex is usable as a Reversible, Standard and Normal Hydrogen Electrode (RHE SHE, NHE). | + | Copper-plated Zensor electrodes have been used to detect various sugars (which is the type of carbohydrates I guess your customers are interested in). See the Zen2005 paper "An electrochemical cell coupled with disposable screen-printed electrodes for use in flow injection analysis". Copper plating of carbon Zensor electrode (eg [https://www.edaq.com/ET083-40 ET083]) is also described in this paper. These electrodes can be used with the [https://www.edaq.com/ET066 Zensor Flow cell] or the customer might be able to build their own flow cell. |
| + | |||
| + | Since then Zensor also produce a 'copper nanoparticle' electrode that is suited for sugar detection, see their 'NCSE' series screen printed working electrodes, (brochure enclosed, in traditional Chinese [[File:Ncse.pdf]]). We don't stock the electrodes but when we last inquired about them they sold in a pack of 8 for the same price as charged for a pack of 40 of the carbon electrodes (ie five times more expensive than ET083). | ||
| + | |||
| + | |||
| + | === O-Rings used by eDAQ === | ||
| + | |||
| + | QUESTION | ||
| + | |||
| + | What is the O-ring material used by eDAQ on various electrodes. | ||
| + | |||
| + | ANSWER | ||
| + | |||
| + | Nitrile/NBR a synthetic rubber used in many critical applications. | ||
| + | https://en.wikipedia.org/wiki/Nitrile_rubber | ||
| + | |||
| + | |||
| + | === Calomel Electrodes === | ||
| + | |||
| + | QUESTION | ||
| + | |||
| + | Does eDAQ sell Calomel electrodes? | ||
| + | For information on calomel electrodes check out our web page at | ||
| + | |||
| + | https://www.edaq.com/wiki/Reference_Electrode_Potentials#The_Calomel_.28Hg.2FHg2Cl2.29_Electrode | ||
| + | |||
| + | |||
| + | ANSWER | ||
| + | |||
| + | No we don't. Because of the many restrictions on selling and shipping mercury containing products (calomel is a mixture of mercury and mercurous chloride) we refer our customers to one of these sellers. | ||
| + | |||
| + | |||
| + | Commercial calomel electrodes are available from: | ||
| + | |||
| + | Koslow Scientific (USA) http://www.koslow.com | ||
| + | ALS Co. Ltd (Japan) https://www.als-japan.com/1390.html | ||
| + | Ionode Pty Ltd (Australia) http://www.ionode.com | ||
| + | |||
| + | The real question is why anyone would want to use a calomel electrode in the first place? If the answer is that they have always done (they were once considered easy to make by the user) then the obvious question is why can't they use a silver/silver chloride electrode. | ||
| + | |||
| + | There may be some technical reason that precludes the use of a silver/silver chloride electrode, and this may need to be verified. But otherwise why not use an off-the-shelf silver/silver chloride electrode, including our leakless reference electrodes - they are usually cheaper and come in a greater variety of shapes and sizes. | ||
| + | |||
| + | |||
| + | === Use of Hydroflex Hydrogen Electrodes === | ||
| + | |||
| + | QUESTION | ||
| + | |||
| + | We have some laboratory electrochemical test cells that need a good reversible hydrogen reference electrode. The working electrolyte for these cells is 32% caustic soda at 90 degrees C. Will the [https://www.edaq.com/ET070 Hydroflex reference electrode] hold up well in these conditions? | ||
| + | |||
| + | ANSWER | ||
| + | |||
| + | Hydroflex is usable as a Reversible, Standard and Normal Hydrogen Reference Electrode (RHE SHE, NHE). | ||
| − | The most common use of | + | The most common use of Hydroflex in the daily lab routine certainly is the application as RHE. You simply dip Hydroflex into your solution, directly. The advantages are obvious. You don't need a liquid junction, you don't have diffusion potentials and you don't contaminate your solution by ions flowing out of your reference system. |
| − | As | + | As Hydroflex needs no maintenance except the regular exchange of the H2-Cartridge every 6 months, it is very well applicable for long-term tests. |
Hydroflex is particularly suitable as a reference electrode in aqueous acid or alkali solutions, and can be used at pressures up to 10 bar and temperatures of up to 210 °C. pH range -2 to pH 16 | Hydroflex is particularly suitable as a reference electrode in aqueous acid or alkali solutions, and can be used at pressures up to 10 bar and temperatures of up to 210 °C. pH range -2 to pH 16 | ||
| − | === | + | QUESTION |
| + | |||
| + | What cleaning is recommended for the Hydroflex Hydrogen Reference Electrode? | ||
| + | |||
| + | ANSWER | ||
| + | |||
| + | Usually when the user exchanges the hydrogen source (cartridge) in time - that means before the hydrogen gas has been exhausted - the reference electrode will not require cleaning. | ||
| + | |||
| + | In the case that the user missed that moment (for example exchanging the cartridge after 8 month instead of the adjusted lifetime of, for example, 6 month) then the user should perform a more complete cleaning as mentioned in the manual. The complete cleaning should be done, as aggressive solutions may attack the metals (Pt, Pd) when no hydrogen is available. | ||
| + | |||
| + | 1. Clean the catalyst with HNO3 for some 3-4 minutes. | ||
| + | 2. Wash out any residuals with water. | ||
| + | 3. Dry the whole electrode in order to get rid of water inside of Hydroflex. | ||
| + | |||
| + | When following this procedure the Hydroflex is usually restored to a "new" condition and should work properly. | ||
| + | |||
| + | |||
| + | === Conductivity Probes ET901 ET902 ET903 Lose the Black Plating === | ||
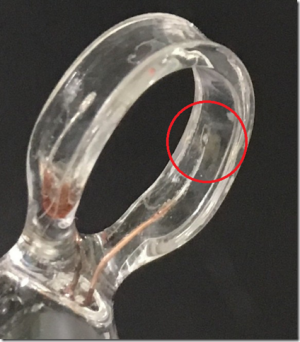
[[File:ET903 Conductivity Probe loses its black plating.png|300px|thumb|right|ET903 Conductivity Probe loses its black plating]] | [[File:ET903 Conductivity Probe loses its black plating.png|300px|thumb|right|ET903 Conductivity Probe loses its black plating]] | ||
| Line 230: | Line 372: | ||
After platinization, the electrode should be rinsed and stored in distilled water. The electrode loses its catalytic properties on prolonged exposure to air and becomes more likely to damage. | After platinization, the electrode should be rinsed and stored in distilled water. The electrode loses its catalytic properties on prolonged exposure to air and becomes more likely to damage. | ||
| − | If some of the platinum black has been removed, the K value will change, but can be adjusted for by calibration. If most has been removed, it will require to be re-platinised | + | If some of the platinum black has been removed, the K value will change, but can be adjusted for by calibration. If most has been removed, it will require to be re-platinised. |
Platinum black on the surface of the electrode is fragile and can be shaken off or damaged and may need recoating. These electrodes are suitable for continuous use in a stable environment. The length of continuous use may depend on the exact conditions of flow/ temperature and mechanical conditions. | Platinum black on the surface of the electrode is fragile and can be shaken off or damaged and may need recoating. These electrodes are suitable for continuous use in a stable environment. The length of continuous use may depend on the exact conditions of flow/ temperature and mechanical conditions. | ||
| Line 236: | Line 378: | ||
The above is relevant for the [https://www.edaq.com/ET901 ET901], [https://www.edaq.com/ET902 ET902] and [https://www.edaq.com/ET903 ET903] conductivity probes. | The above is relevant for the [https://www.edaq.com/ET901 ET901], [https://www.edaq.com/ET902 ET902] and [https://www.edaq.com/ET903 ET903] conductivity probes. | ||
| − | + | '''Instructions for replatinization of electrode surfaces using a YSI 3139 PLATINIZING KIT''' | |
| − | + | The kit consists of a 4.5 VDC supply containing three “D” (flashlight) cells, a | |
| + | milliammeter, a current control, and a polarity reversal switch. A 3 ½ oz. Jar is included | ||
| + | for use as a platinizing solution container. | ||
| + | Platinizing solution is not included with the kit. It is available in 2 oz. quantities – | ||
| + | enough to platinize at least 25 cells – as YSI No. 3140. This solution consists of 1.77 | ||
| + | gm Platinic Chloride and .015 gm Lead Acetate dissolved in 2 oz. distilled water. | ||
| − | + | '''OPERATION''' | |
| − | + | 1. Place the solution container in the clip provided on the instrument. | |
| + | 2. Pour 2 oz. platinizing solution into the container. | ||
| + | 3. Place the cell in the solution and connect the cell leads to the binding posts. | ||
| + | 4. Adjust the current control for 50 MA indication on the meter, tap meter. | ||
| + | 5. Platinize for 3 to 4 minutes. Reverse the polarity ever 30 seconds. | ||
| + | 6. Rinse the cell in running water for about 15 minutes. | ||
| + | 7. Rinse the cell in distilled water. | ||
| − | + | '''CELL CLEANING''' | |
| − | + | It may be necessary to clean the cell in order to ensure a good coating of | |
| + | platinum black. Since the electrodes in the YSI 3400 Series Cell are soldered with fine | ||
| + | gold, DO NOT CLEAN THIS CELL IN AQUA REGIA OR IN SOLUTIONS WHICH MIGHT ATTACK GOLD. | ||
| − | + | The conductivity cell should be cleaned as follows: | |
| − | + | 1. Prepare a solution containing 100 ml isopropyl alcohol, 50 ml concentrated HCl, and | |
| + | 50 ML distilled water. | ||
| + | 2. Immerse the cell electrode chamber in the solution for 3 to 4 minutes. | ||
| + | 3. Rinse the cell in distilled water. | ||
| − | |||
| − | + | === ET1120 Oxygen electrode Cleaning and storage === | |
| − | + | CLEANING | |
| − | + | Using any electrode in solutions containing protein requires the electrode be rinsed with an enzyme cleaning solution. | |
| + | After each use, we recommend cleaning our electrodes with Terg-a-zyme (Alconox, Inc.) or a chromic/sulfuric acid glass cleaning solution by submerging the electrodes for a couple of minutes in order to remove all protein from the glass and reference junction. | ||
| + | This will prolong the useful life of the electrodes. | ||
| − | + | STORAGE | |
| − | + | Always clean the microelectrode before storing: | |
| + | * Long-term (over 2 weeks): Return the probe to its original container and prepare it in the same condition in which you received it. Usually this means simply moistening the sponge located in the bottom of the protective glass tube with pH 4 buffer. | ||
| + | * Short-term: The probe can be left in an acid pH buffer solution (pH 4.01). | ||
| − | |||
| − | + | === ET05x ET07x Electrodes Metal purity === | |
| − | + | All metals used in the construction of ET05x and ET07x electrodes have a purity level of 99.99%. The next step is 99.999% for ultra pure metals - there is no need to go further than 99.99% for electrodes. | |
| − | + | As an example for copper: | |
| + | |||
| + | Alloy 101 OFE Copper is the highest purity grade of copper at 99.99%. OFE stands for oxygen-free electrolytic and replaces the acronym OFHC. It has been electrolytically refined in a carefully regulated, oxygen-free environment to reduce the level of oxygen to .001% or below. | ||
| + | |||
| + | === What is the minimum sample volume required ? === | ||
| + | |||
| + | As long as the electrode tip is fully submerged in solution. This depends on the electrode cell. A small vial with conical shaped bottom is recommended. | ||
| + | |||
| + | https://www.edaq.com/ET080-12 | ||
| + | |||
| + | The other possibility is to use the electrode upside down. Just a droplet of sample will do. However, the tricky issue is to position the reference and counter electrode in the same sample droplet. | ||
Latest revision as of 11:00, 6 October 2023
You can learn more about the electrodes by looking at our range of electrodes, the relevant research sections, and for voltammetric electrodes: the videos, and application notes.
Contents
- 1 ET069 and ET072 Leakless Ag/AgCl Reference Electrodes
- 2 Other Electrodes
- 2.1 When do Electrodes require cleaning?
- 2.2 ET073 Use in Organic Solvents
- 2.3 Measuring Sugars using Zensor Electrodes
- 2.4 O-Rings used by eDAQ
- 2.5 Calomel Electrodes
- 2.6 Use of Hydroflex Hydrogen Electrodes
- 2.7 Conductivity Probes ET901 ET902 ET903 Lose the Black Plating
- 2.8 ET1120 Oxygen electrode Cleaning and storage
- 2.9 ET05x ET07x Electrodes Metal purity
- 2.10 What is the minimum sample volume required ?
ET069 and ET072 Leakless Ag/AgCl Reference Electrodes
General
General Notes on Use
Leakless electrodes ET069 and ET072
- DO NOT APPLY TOO MUCH PRESSURE ON THE CONNECTOR PIN.
- Remove the cap protecting the tip before use by gripping the electrode by its PEEK body and carefully removing the plastic cap.
The electrode utilizes a unique junction which is robust and highly conductive but not porous. There is no glass used in the construction.
The electrode cannot be refilled.
The electrode is not affected by hydrofluoric acid and common dilute acids, and bases. It is resistant to most commonly used organic solvents. If the electrode is left to dry for a very long period of time, it should be immersed in deiniozed water for a few hours before use.
Read instructions regarding Long Term storage of electrodes.
If using the electrode in solutions containing ions that form precipitates with chloride and/or potassium ions, then DO NOT store the electrode in potassium chloride solution.
If using the electrode in dry organic solvent, the electrode should be rinsed with acteone (to remove water), then rinsed with the final solvent. The electrode should be stored in 0.05-0.1 M sulfuric acid, NOT the organic solvent when not in use.
Depending on the choice of solvent, substrate molecules, and level of care, the electrode should last many months if not several years.
An old electrode suffering from potential drift can sometimes be reactivated by subjecting it to a large oxidizing potential (+4 V) in a two electrode system (use a wire for the counter electrode) in a KCl solution for 10 –15 seconds then waiting 30 seconds for stabilization. Material adsorbed on the electrode surface can be removed by careful polishing on fine sand paper (or with abrasive powder). Alternatively, try immersing in strong acid (e.g. 6 mol/L H2SO4) for 30 minutes then sonicate, and repeat if necessary.
Download the pdf Instruction Sheet from: File:ET069 ET072 Instruction Sheet.pdf
General Questions
> 1. what is the resistance of the leakless ref electrode ?
less than 10 kohm
> 2. is there a difference between the miniature and the larger LF electrodes (aside from the size) ?
No.
> 3. is there any experience with use of these LF electrodes used over months or even years (drift of potential due to ions intruding from the electrolyte?)
Depending on conditions (solvents, temperature, etc) and frequency of use you can usually expect months to years of use.
> 4. what is the pin material ?
gold plated
> 5. What is the maximum operating temperature.
The electrode will operate at temperatures below 90 Centigrade. Exceeding this temperature can cause boiling of the internal solution leading potentially to electrode damage.
> 6. Electrodes showing drift Customer reported drift of 50mV after a week of use in a fixed 7.5pH environment.
ANSWER: The electrode can handle extreme cases of acid and bases. However there might be some material aadsorbed on the electrode surface. Please dip in 0.5-1M sulfuric acid and/or 1M hydroxide for a few minutes and then acetone or ethanol for additional cleaning. If the electrode has been left dry for some time, soak for a few hours in water.
> 7. The ET072 electrode is characterized by its leakless property. The application note says that the filling electrolyte (3.4 M KCl) doesn’t leak into the sample. Without ion exchange, how does the charge transfer achieve?
ANSWER: The junction was formulated to provide conductivity without the need to leak potassium and chloride ions.
> 8. Would there be any problem if ET072 is used in electrolytes with low ion concentration? One of my experiments use DI water as the electrolyte.
ANSWER: No problem. you can use it in pure water.
> 9. Will the ET072/ET069 electrode be OK for use in the organic solvent tributyl phosphate (TBP)? I would like to use it in TBP that also will probably have nitric acid (HNO3) in it in 0.5 M to 3 M concentration. There will be some water in this; it will not be dry. Will the electrode be OK in this system?
ANSWER: Yes, it can be used in (TBP). The electrodes are resistant to nitric acid. We tried higher concentrations and did not damage them.
> 10. Recently we purchased some small custom electrodes that were packed with a small vial to ensure electrodes don't dry out during shipping. Customer is asking "What is the liquid ?
ANSWER: It is diluted, about 0.05 M sulfuric acid with little potassium chloride. Concentrations are not crucial. Frequent users of the 1 mm OD, claim they perform better when shipped in vials. Some times we ship the LF-1.6 in vials.
> 11. I am currently using your ET072 leakless Ag/AgCl reference electrode and it works really nice. However, the temperature range is stated between 0-90 C, we are planning to do tests with temperatures up to 140 C, do you have any reference electrodes with the same dimensions as the ET072 that handles temperatures that high?
ANSWER: If the user going to use the electrode over short periods of time, say 30 min each time, the junction and PEEK body can handle that. The only worry is the boiling of the filling solutionmay cause damage the connector seal. The user can immerse just 5 to 10 mm of the tip in the heated solution if possible. Ask the user to try it. If damaged we can replace and think of a different method of preparation such as leaving little room inside the electrode for expansion. Remember that we can make very long electrodes if the solution container is deep or make electrodes bent at certain angles.
> 12. Your website and documentation is unclear on what the storage solution for this electrode should be. In one place, it says "aqueous solution" while in another place it says "distilled water" and in a final place it says "0.05 to 0.1 M sulfuric acid". Could you clarify which storage solution I should use for my application?
ANSWER: They can be stored in distilled water if needed, or the diluted sulfuric acid. Indeed, any aqueous solution should be fine. Just the 0.05 M sulfuric is recommended but NOT a must.
>13. The ET072 electrode is characterized by its leakless property. The application note says that the filling electrolyte (3.4 M KCl) doesn’t leak into the sample. Without ion exchange, how does the charge transfer achieve?
ANSWER: The junction was formulated to provide conductivity without the need to leak potassium and chloride ions.
>14. Would there be any problem if ET072 is used in electrolytes with low ion concentration? One of my experiments use DI water as the electrolyte. If the ET072 is used as the reference electrode in such experiments, is it possible to estimate its equilibrium potential through calculation?
ANSWER: No problem. you can use it in pure water. This Ag/AgCl/KCl electrode is about 224 mV vs standard hydrogen electrode.
>15. I would like to ask you about the potential of reference electrode vs. SHE.
ANSWER: It is about 224 mV vs SHE.
>16. Do you have any information about the compatibility of the AMANI1600 Micro pH sensor with a Mettler Toledo pH meter, which uses a sensor like this: https://www.mt.com/gb/en/home/products/Laboratory_Analytics_Browse/pH-meter/sensor/pH-sensor/InLab-Routine.html
ANSWER: Our pH sensors come with a BNC connector. So they compatible with any pH meter or potentiometer that accepts a BNC connection. What is the connection on the meter, not the sensor, because the sensor in the link screws to another end or socket which connects it to meter.
>17. For my research I have to build my CV cells in the glovebox and therefore vacuum is applied on my ET072-1 electrodes. Thus, I want to know how vacuum resistant these electrodes are or if they get broken during vacuum application?
ANSWER: The electrode should be fine in vacuum. The ET-72 ( LF-2) has a thick wall and is completely sealed. The user should try it and if the electrode damaged "explodes" we can replace or refund it. I am sure over the years researchers have used ET072 under such conditions and I did not hear a complaint.
>18. Can you please advise me if ET072/ET069 can be used with Toluene and dibenzyltoluen ?
ANSWER: Yes, it can be used. I do not expect any problem.
Storage
Long term storage
Leakless electrodes ET069 and ET072 can be stored for both short and long term duration's in distilled water, or the diluted sulfuric acid, 0.05 to 0.1 M sulfuric acid. Indeed, any aqueous solution should be fine. Just the 0.05 M sulfuric is recommended but NOT a must. Based on this storage method the electrodes should last for many months if not several years. Because eDAQ has no control over the use of these electrodes our warranty is limited to 3 months from date of invoice.
Please remember these electrodes are designed primarily for ease of use, and generally need to be replaced when they begin to show excessive drift. See information sheet at
https://www.edaq.com/product_sheets/transducers/ET072_Leakless_Miniature_Ag-AgCl_Reference_Electrode.pdf. for Maintenance details.
Use and Solvents
Effect of high pH values
QUESTION
A customer would like to know if the ET072 Leakless Miniature Ag/AgCl Reference Electrode is stable in extreme pH-conditions. It would be used for several weeks in pH 12-13 environments. Would that be a problem you think ?
ANSWER
The LF electrodes were kept in 5 M potassium hydroxide for few days and in 3 M in sodium hydroxide for over a year. No junction damage occurred. A little shift in potential might occur, but the electrode functions well
Extreme operating conditions
QUESTION
- A customer would like to know if the ET072 Leakless Miniature Ag/AgCl Reference Electrode will endure 1M KOH solution @ 80°C conditions ?
- Is your ET074 glassy carbon electrodes heat-resistant and work up to 140 degrees Celsius?
ANSWER
- ET072 was boiled in KOH for 15 min then left it to cool down for one hour in KOH. There were no apparent change in conductivity or potential.
Soaked one ET072 in 5 M KOH at room temperature for weeks. There was no damage.
This is an important advantage, since researchers use toxic mercury/mercury oxide electrodes with porous junctions because normal Ag/AgCl electrodes are not stable due to the formation of Ag(OH) which is converted to Ag2O. So our ET072 electrode can be used in extreme acid or extreme base. These electrodes have been soaked for long periods >300days and even boiled in 100g/l Sulfuric acid without damage.
- ET074 Glassy Carbon voltammetric disk electrode, will operate at temperatures below 90 Centigrade. Exceeding this temperature can cause electrode damage.
High resistance of ET072
QUESTION
I recently purchased a miniature leakless Ag/AgCl reference electrode from eDAQ, and I am trying to use it for cyclic voltammetry in a rotating disk electrode setup. I am getting very bizarre results and my potentiostat is having trouble giving me a quality uncompensated resistance value for the cell (gives high phase error). I did not have this problem when working with an Ag/AgCl with a porous junction. Is the resistance of these leakless electrodes necessarily higher than that of those with porous frits? Is there a difference in experimental applications between your miniature leakless electrodes and regular-sized ones (i.e., is there a range of suitable currents for the smaller electrode vs. the larger one)?
ANSWER
The ET072 has an internal resistance (impedance) of less than 10 kohm. In most cases this will not present a problem for the potentiostat however in some cases large electrode impedance (depending on factors such as choice of electrolyte solution, distances between working, reference, and auxiliary electrodes, etc) may cause potentiostat instability, especially if positive feedback iR compensation is being used.
In such cases it may be necessary to:
1. run the potentiostat in 'high stability' mode (refer to the potentiostat manual)
2. select a different reference electrode with lower impedance. The 'leakier' the electrode the less resistance/impedance it will have.
3. change the electrochemical cell design and especially bring the electrodes closer together,
4. increase the concentration of the background electrolyte, or
5. introduce a capacitor of appropriate size between the reference and auxiliary electrode.
Use in Ionic Liquids
QUESTION
Can ET069 and ET072 leakless ref electrodes be used in ionic liquids (RTILs Room Temperature Ionic Liquids)?
ANSWER
These electrodes should be OK to use in most ionic liquids. However the potentials under these conditions are not established standards and it would be best at the end of the experiment to use a cyclic voltammogram of ferrocene in the ionic liquid to determine reportable values. Ferrocene is also often used as a reference for volumetric experiments in organic solvents.
Use in aqueous solutions of Bases and Acids
QUESTION
Is it possible to use ET072 and ET069 electrodes in diluted HCLO4 (aqueous solution at ~2M) during several hours? Is it possible to use it in diluted HF solutions (aqueous solution up to 5M) ? - within which pH range (aqueous solution) can it be used?
ANSWER
The electrode material is not affected by the acids mentioned above. This was established years ago. The electrodes can handle 5M acid or 5M base. There might be a small shift in potential which is reversible but no physical damage or leakage occurs. Can be used over the full range of pH and temperatures
Use in aggressive solvents
QUESTION
Can the ET072 and ET069 leakless electrodes be used in organic solvents, perchlorate and silver salts solutions, Dimethylformamide or Hydrofluoric acids?
ANSWER
Our leakless electrodes ET072 and ET069 enable you to perform your experiments in organic solvents, perchlorate and silver salts solutions, Dimethylformamide or Hydrofluoric acids without being worried about clogging or degradation! It can also be used for long term experiments without the worry that the filling electrolyte be diluted or run out. This leakless reference electrode uses our newly developed conductive junction. The filling electrolyte is confined to the barrel and will not leak at all (zero leakage). The junction has very high conductivity with resistance under 10 kohm. It has exceptional mechanical stability, zero swelling, resistance to organic solvents, and is robust. The junction potential is independent of the sample nature or ionic strength. The electrode body is constructed from PEEK for superior chemical resistance. The filling electrolyte (3.4 M KCl) does not leak through the junction which prevents sample contamination with chloride and potassium ions. This means no clogging and no need for double junction. Since the electrode construction does not involve any glass, it can be used in hydrofluoric acid solutions.
Other Electrodes
When do Electrodes require cleaning?
See the application note Cleaning and Polishing Voltammetric Electrodes
ANSWER
1. If they look dirty they probably are and cleaning should be done.
2. If the electrochemistry reaction produces any sort of insoluble material (including any sort of electrodeposition or electropolymerization reaction) then cleaning of the working electrode (and maybe also the auxiliary electrode) will be required.
3. If you get strange peaks in a cyclic voltammogram run when the the electrodes are placed in fresh solvent/electrolyte then (assuming the electrolyte solution is pure) then the working electrode surface should be cleaned.
4. If the current is unexpectedly small then the working (or auxiliary) electrode surface may be coated with a non conductive material. Although by this stage it would normally be visibly fouled.
5. If you get the expected voltammetric peaks but at wrong E values then the reference electrode may be exhausted and need regenerating or replacement.
6. If you get oscillations/noise in starting a volumetric experiment then the reference electrode may be clogged or broken giving an open circuit. If this is suspected then repeat the experiment without the reference electrode attached. If you get a similar result then the reference electrode needs cleaning or replacement.
Our ET030 Electrode Polishing Kit is useful for cleaning electrodes.
ET073 Use in Organic Solvents
Question: I want to use the eDAQ ET073 Refillable Miniature Ag/AgCl Reference Electrode in water-free conditions. Therefore, I filled it with AgNO3 (0.1M) in ACN. However, the potential of this Ag/AgNO3 reference electrode is not constant. Should I remove the darker AgCl coating from the silver wire?
Answer provided by Dr Paul Duckworth:Customer is attempting to make a silver/silver ion electrode for use in organic solvents (in the case 'AN' acetonitrile).
To do this the AgCl coating on the silver wire MUST be completely removed. This can be done by using abrasive paper to rub the AgCl coating off. You can also use 1 mol/L ammonia solution to dissolve the AgCl.
You can then fill the ET073 electrode with 0.1 mol/L silver salt solution (usually silver nitrate, tetrafluroborate, or hexafluorophosphate).
You should now get a steady potential (but you need to keep temperature constant, to at least within 1 centigrade degree, to keep the potential constant to within 1 mV).
Note that this type of reference electrode must not be used in a solution that contains ions like Cl-, Br-, I-, SCN-, OH-, S2-, or any other ion that will react with Ag+ ion to form a precipitate.
Measuring Sugars using Zensor Electrodes
QUESTION
Customer asked about measuring total carbohydrates (Sugars in sweet beverages)
ANSWER
Copper-plated Zensor electrodes have been used to detect various sugars (which is the type of carbohydrates I guess your customers are interested in). See the Zen2005 paper "An electrochemical cell coupled with disposable screen-printed electrodes for use in flow injection analysis". Copper plating of carbon Zensor electrode (eg ET083) is also described in this paper. These electrodes can be used with the Zensor Flow cell or the customer might be able to build their own flow cell.
Since then Zensor also produce a 'copper nanoparticle' electrode that is suited for sugar detection, see their 'NCSE' series screen printed working electrodes, (brochure enclosed, in traditional Chinese File:Ncse.pdf). We don't stock the electrodes but when we last inquired about them they sold in a pack of 8 for the same price as charged for a pack of 40 of the carbon electrodes (ie five times more expensive than ET083).
O-Rings used by eDAQ
QUESTION
What is the O-ring material used by eDAQ on various electrodes.
ANSWER
Nitrile/NBR a synthetic rubber used in many critical applications. https://en.wikipedia.org/wiki/Nitrile_rubber
Calomel Electrodes
QUESTION
Does eDAQ sell Calomel electrodes? For information on calomel electrodes check out our web page at
https://www.edaq.com/wiki/Reference_Electrode_Potentials#The_Calomel_.28Hg.2FHg2Cl2.29_Electrode
ANSWER
No we don't. Because of the many restrictions on selling and shipping mercury containing products (calomel is a mixture of mercury and mercurous chloride) we refer our customers to one of these sellers.
Commercial calomel electrodes are available from:
Koslow Scientific (USA) http://www.koslow.com ALS Co. Ltd (Japan) https://www.als-japan.com/1390.html Ionode Pty Ltd (Australia) http://www.ionode.com
The real question is why anyone would want to use a calomel electrode in the first place? If the answer is that they have always done (they were once considered easy to make by the user) then the obvious question is why can't they use a silver/silver chloride electrode.
There may be some technical reason that precludes the use of a silver/silver chloride electrode, and this may need to be verified. But otherwise why not use an off-the-shelf silver/silver chloride electrode, including our leakless reference electrodes - they are usually cheaper and come in a greater variety of shapes and sizes.
Use of Hydroflex Hydrogen Electrodes
QUESTION
We have some laboratory electrochemical test cells that need a good reversible hydrogen reference electrode. The working electrolyte for these cells is 32% caustic soda at 90 degrees C. Will the Hydroflex reference electrode hold up well in these conditions?
ANSWER
Hydroflex is usable as a Reversible, Standard and Normal Hydrogen Reference Electrode (RHE SHE, NHE).
The most common use of Hydroflex in the daily lab routine certainly is the application as RHE. You simply dip Hydroflex into your solution, directly. The advantages are obvious. You don't need a liquid junction, you don't have diffusion potentials and you don't contaminate your solution by ions flowing out of your reference system. As Hydroflex needs no maintenance except the regular exchange of the H2-Cartridge every 6 months, it is very well applicable for long-term tests.
Hydroflex is particularly suitable as a reference electrode in aqueous acid or alkali solutions, and can be used at pressures up to 10 bar and temperatures of up to 210 °C. pH range -2 to pH 16
QUESTION
What cleaning is recommended for the Hydroflex Hydrogen Reference Electrode?
ANSWER
Usually when the user exchanges the hydrogen source (cartridge) in time - that means before the hydrogen gas has been exhausted - the reference electrode will not require cleaning.
In the case that the user missed that moment (for example exchanging the cartridge after 8 month instead of the adjusted lifetime of, for example, 6 month) then the user should perform a more complete cleaning as mentioned in the manual. The complete cleaning should be done, as aggressive solutions may attack the metals (Pt, Pd) when no hydrogen is available.
1. Clean the catalyst with HNO3 for some 3-4 minutes. 2. Wash out any residuals with water. 3. Dry the whole electrode in order to get rid of water inside of Hydroflex.
When following this procedure the Hydroflex is usually restored to a "new" condition and should work properly.
Conductivity Probes ET901 ET902 ET903 Lose the Black Plating
QUESTION
Please see the photo of ET903 electrode. When we first delivered the electrode to our user, the red marked platinum plate was plated by some black substance. But now the black substance peels off from the platinum plate.
Please advise us whether we can use the electrode continuously or not.
ANSWER
These electrodes or probes use platinum which is platinised. See this Wikipedia explanation
After platinization, the electrode should be rinsed and stored in distilled water. The electrode loses its catalytic properties on prolonged exposure to air and becomes more likely to damage.
If some of the platinum black has been removed, the K value will change, but can be adjusted for by calibration. If most has been removed, it will require to be re-platinised.
Platinum black on the surface of the electrode is fragile and can be shaken off or damaged and may need recoating. These electrodes are suitable for continuous use in a stable environment. The length of continuous use may depend on the exact conditions of flow/ temperature and mechanical conditions.
The above is relevant for the ET901, ET902 and ET903 conductivity probes.
Instructions for replatinization of electrode surfaces using a YSI 3139 PLATINIZING KIT
The kit consists of a 4.5 VDC supply containing three “D” (flashlight) cells, a milliammeter, a current control, and a polarity reversal switch. A 3 ½ oz. Jar is included for use as a platinizing solution container. Platinizing solution is not included with the kit. It is available in 2 oz. quantities – enough to platinize at least 25 cells – as YSI No. 3140. This solution consists of 1.77 gm Platinic Chloride and .015 gm Lead Acetate dissolved in 2 oz. distilled water.
OPERATION
1. Place the solution container in the clip provided on the instrument. 2. Pour 2 oz. platinizing solution into the container. 3. Place the cell in the solution and connect the cell leads to the binding posts. 4. Adjust the current control for 50 MA indication on the meter, tap meter. 5. Platinize for 3 to 4 minutes. Reverse the polarity ever 30 seconds. 6. Rinse the cell in running water for about 15 minutes. 7. Rinse the cell in distilled water.
CELL CLEANING
It may be necessary to clean the cell in order to ensure a good coating of platinum black. Since the electrodes in the YSI 3400 Series Cell are soldered with fine gold, DO NOT CLEAN THIS CELL IN AQUA REGIA OR IN SOLUTIONS WHICH MIGHT ATTACK GOLD.
The conductivity cell should be cleaned as follows:
1. Prepare a solution containing 100 ml isopropyl alcohol, 50 ml concentrated HCl, and 50 ML distilled water. 2. Immerse the cell electrode chamber in the solution for 3 to 4 minutes. 3. Rinse the cell in distilled water.
ET1120 Oxygen electrode Cleaning and storage
CLEANING
Using any electrode in solutions containing protein requires the electrode be rinsed with an enzyme cleaning solution. After each use, we recommend cleaning our electrodes with Terg-a-zyme (Alconox, Inc.) or a chromic/sulfuric acid glass cleaning solution by submerging the electrodes for a couple of minutes in order to remove all protein from the glass and reference junction. This will prolong the useful life of the electrodes.
STORAGE
Always clean the microelectrode before storing:
- Long-term (over 2 weeks): Return the probe to its original container and prepare it in the same condition in which you received it. Usually this means simply moistening the sponge located in the bottom of the protective glass tube with pH 4 buffer.
- Short-term: The probe can be left in an acid pH buffer solution (pH 4.01).
ET05x ET07x Electrodes Metal purity
All metals used in the construction of ET05x and ET07x electrodes have a purity level of 99.99%. The next step is 99.999% for ultra pure metals - there is no need to go further than 99.99% for electrodes.
As an example for copper:
Alloy 101 OFE Copper is the highest purity grade of copper at 99.99%. OFE stands for oxygen-free electrolytic and replaces the acronym OFHC. It has been electrolytically refined in a carefully regulated, oxygen-free environment to reduce the level of oxygen to .001% or below.
What is the minimum sample volume required ?
As long as the electrode tip is fully submerged in solution. This depends on the electrode cell. A small vial with conical shaped bottom is recommended.
The other possibility is to use the electrode upside down. Just a droplet of sample will do. However, the tricky issue is to position the reference and counter electrode in the same sample droplet.