Belousov-Zhabotinsky Oscillating Chemical Reactions
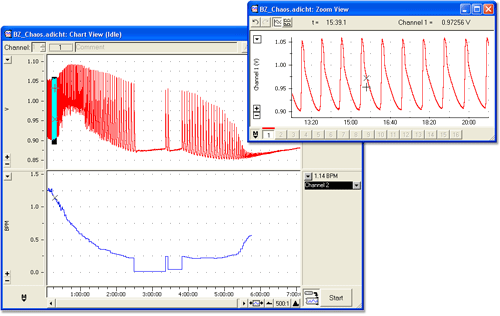
A great demonstration to show that not all chemical reactions follow simple kinetics. Using specific proportions of malonic acid, sodium sulfate and cerric ammonium nitrate in sulfuric acid media, a series of competing and oscillating reaction steps develop whilst the reaction establishes equilibrium.
The oscillations are indicated by color changes, and can last several hours. Using the pH Pod, the oscillations could be monitored electrochemically with a redox electrode or bromide ion selective electrode (or both). The change in frequency and period of the oscillations could be easily displayed using the Cycle Variables extension of Chart.
Keywords: Potentiometry. Kinetics. Chaotic Processes.



